Genomics/Epigenomics
Genomics/Epigenomics
168 - The Role of Hydroxymethylation in Bronchopulmonary Dysplasia
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 168
Publication Number: 168.218
Publication Number: 168.218
Gal Barbut, University of Rochester Medical Center, Rochester, NY, United States; Huiling Li, Baylor College of Medicine, Houston, TX, United States; Jonathan Davies, Baylor College of Medicine, Houston, TX, United States

Gal Barbut, MD
Assistant Professor
University of Rochester Medical Center
Rochester, New York, United States
Presenting Author(s)
Background: Bronchopulmonary dysplasia (BPD) is one of the most frequent and important morbidities for infants born prematurely. Hyperoxia and other noxious stimuli lead to BPD but the specific cellular control leading to this pathophysiology remains unclear. DNA methylation to silence expression is the most well-known epigenetic modification however more recent studies have found hydroxymethylation through Ten-Eleven Translocation (TET) leads to increased DNA expression. The role of hydroxymethylation in the development of BPD remains unknown. We hypothesis that exposure of the developing lung to hyperoxia leads to DNA hydroxymethylation (5hmC) by TET, and these epigenetic changes contribute to the development of BPD.
Objective: To determine the role of hydroxymethylation in the development of BPD by confirming the presence of BPD after exposure to hyperoxia, determining the quantity of TET enzymes and assessing the changes in global hydroxymethylation in our hyperoxia exposed animal groups vs our normoxia controls.
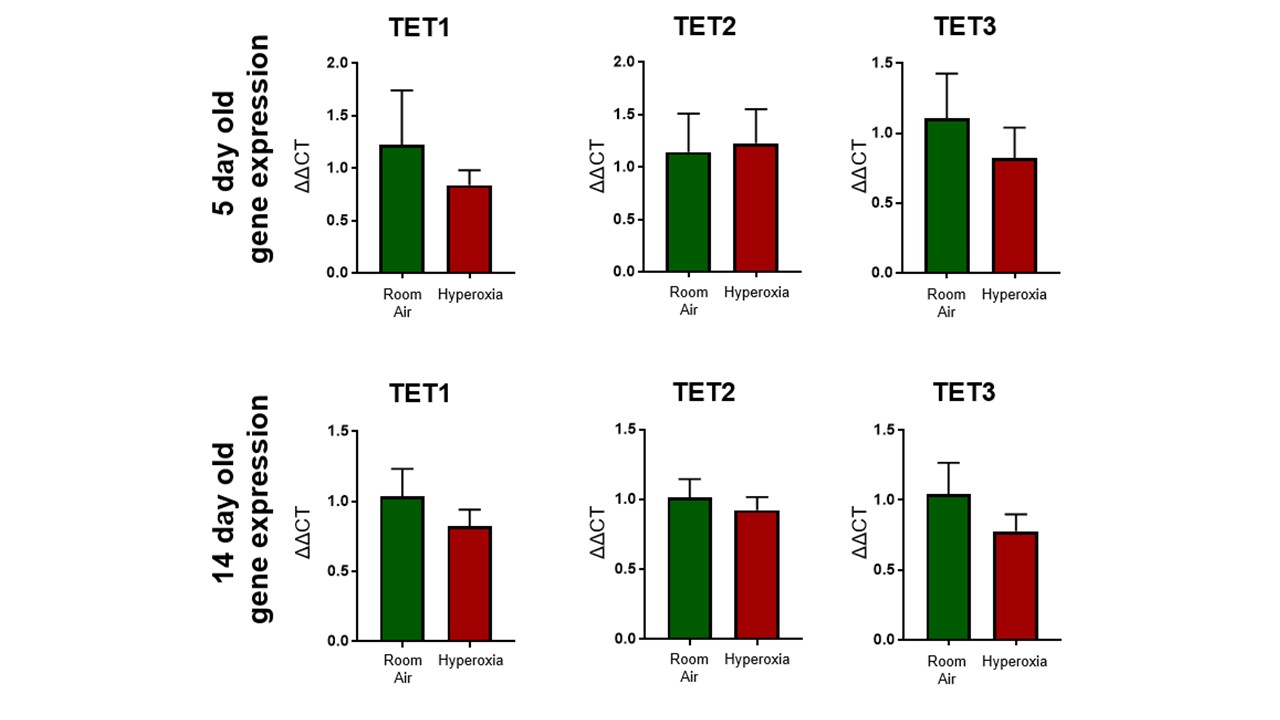
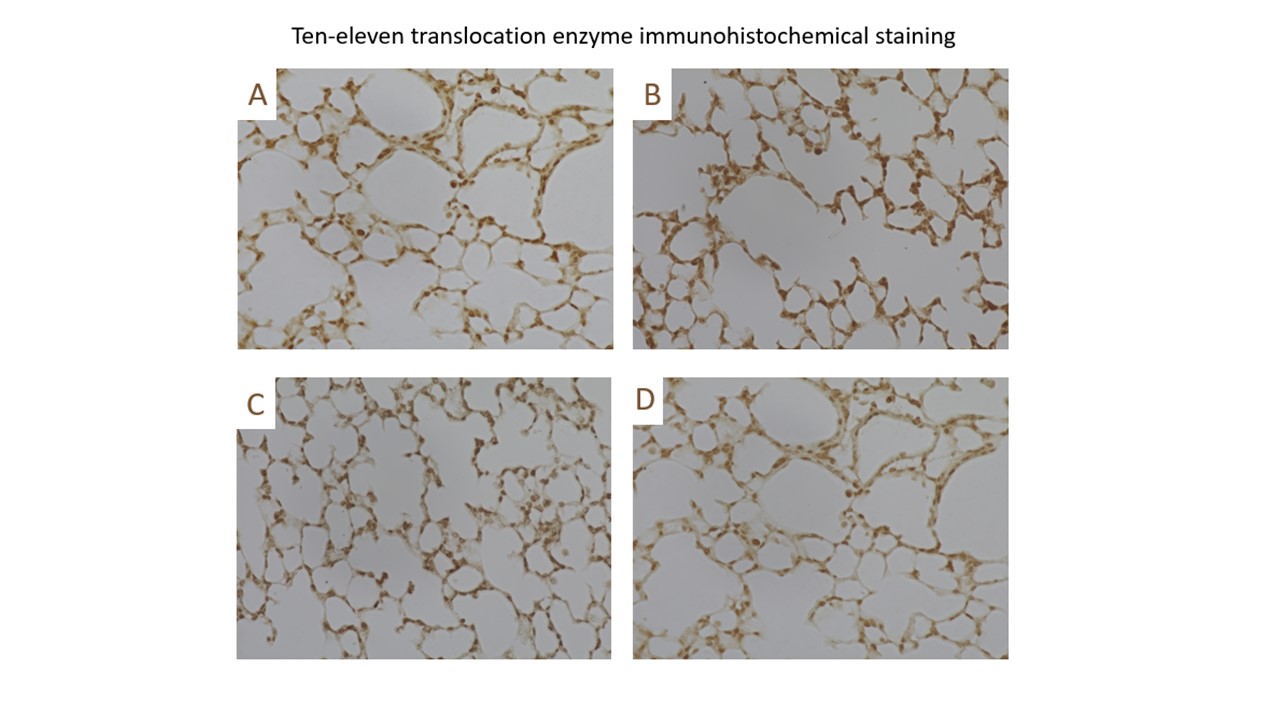
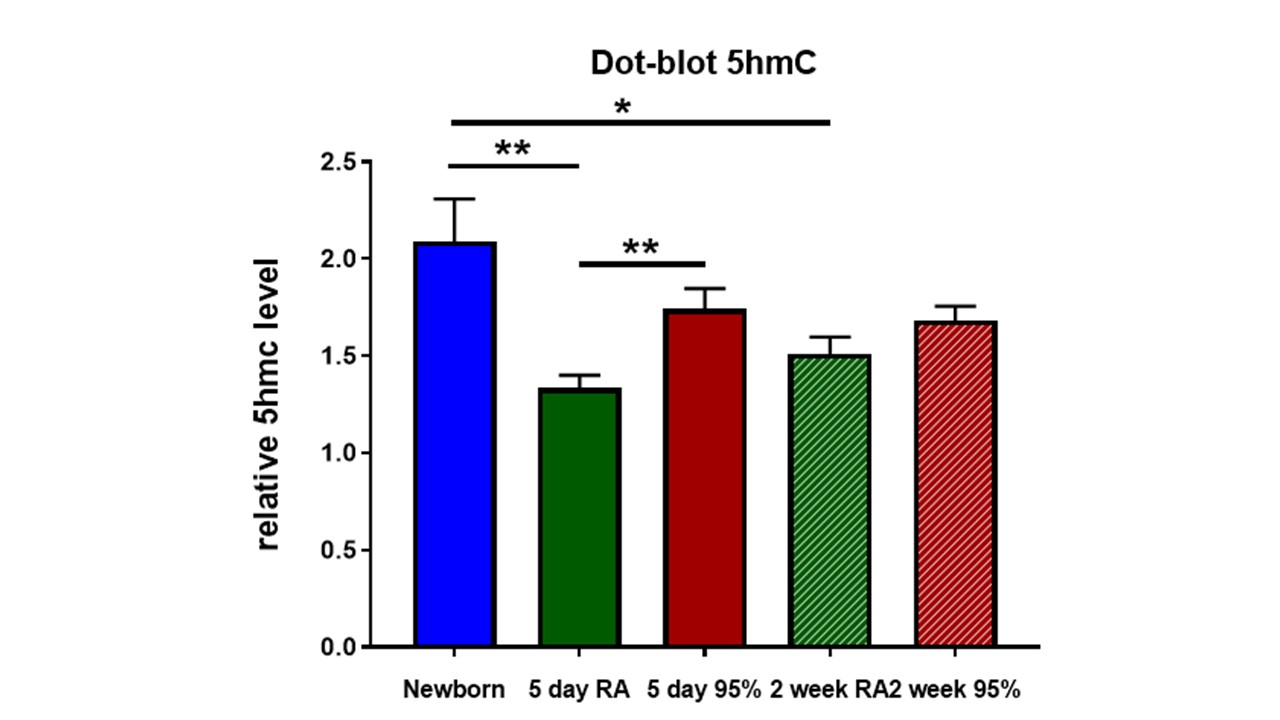
Design/Methods: Newborn C57Bl/6 mice were placed in 95% oxygen for 5 days and recovered in 21% O2. Mice were sacrificed at birth, PN5, and PN14. H&E samples were compared to assess alveolar simplification. TET RNA levels were assessed using quantitative PCR and compared between the two groups. TET protein levels were qualitatively assessed using immunohistochemistry. Global DNA hydroxymethylation levels were assessed using cytosine-5-methylenesulfonate immunoprecipitation (CMS-IP) sequencing and compared between the hyperoxia group and room air controls at the listed time points.
Results: The BPD phenotype was seen in the hyperoxia exposed animals. The TEN enzyme transcript levels were quantitatively unchanged in hyperoxia exposed vs room air control animals with enzyme protein levels qualitatively observed in both samples on immunohistochemistry. The levels of hydroxymethylation were higher in the hyperoxia exposed animals. The highest levels of hydroxymethylation were seen immediately after birth. There was a significant drop in hydroxymethylation in the room air control group whereas the hyperoxia exposed animals had persistently increased hydroxymethylation levels.
Conclusion(s): We conclude the disruption of normal alveolar development in response to hyperoxia correlates with increased hydroxymethylation levels demonstrating an increased activity of the dioxygenase enzyme in a response to oxygen exposure. This finding ultimately supports our initial hypothesis and suggests a potential role for hydroxymethylation in the pathophysiology of BPD.