Genomics/Epigenomics
Genomics/Epigenomics
174 - Association between genetic ancestry and urinary metabolites of extremely premature infants
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 174
Publication Number: 174.218
Publication Number: 174.218
THAYBETH I. MALAVE-MENDEZ, University of California, San Francisco, SAN FRANCISCO, CA, United States; Miguel A. Guardado, UCSF, San Francisco, CA, United States; Cheryl Chapin, UCSF Benioff Children's Hospital San Francisco, San Francisco, CA, United States; Philip Ballard, UCSF Benioff Children's Hospital San Francisco, San Francisco, CA, United States; Dara Torgerson, University of California, San Francisco, School of Medicine, San Francisco, CA, United States; Ryan Hernandez, UCSF, San Francisco, CA, United States; Martina Steurer, UCSF Benioff Children's Hospital San Francisco, San Francisco, CA, United States

THAYBETH I. MALAVE-MENDEZ, BS (she/her/hers)
Graduate Student
University of California, San Francisco
SAN FRANCISCO, California, United States
Presenting Author(s)
Background: The metabolome may be influenced by genetic factors, including genetic variation that is linked to continental genetic ancestry. The contribution of genetic ancestry to the urinary metabolome remains poorly understood.
Objective: Identify metabolites that associate with genetic ancestry independent of race/ethnicity in the urine of premature infants at high risk of bronchopulmonary dysplasia (BPD).
Design/Methods: Untargeted metabolomics was performed using UHPLC/MS-MS on urine samples of 171 infants from TOLSURF < 28 wk gestational age collected at two timepoints between days 6-14 and 23-30 postnatal age. Maternal self-reported race/ethnicities included Hispanic White, non-Hispanic White, or Black/African American. Ancestry proportions were inferred from >800,000 genotypes assuming a 3-population model of continental African, European, and Amerindigenous ancestry. We then tested for an association between proportions of each genetic ancestry and quantitative measures of individual metabolites using linear regression, adjusting for maternal race/ethnicity, sex, birthweight, TPN status, BPD, and gestational age. Analyses were also performed separately within each racial/ethnic group.
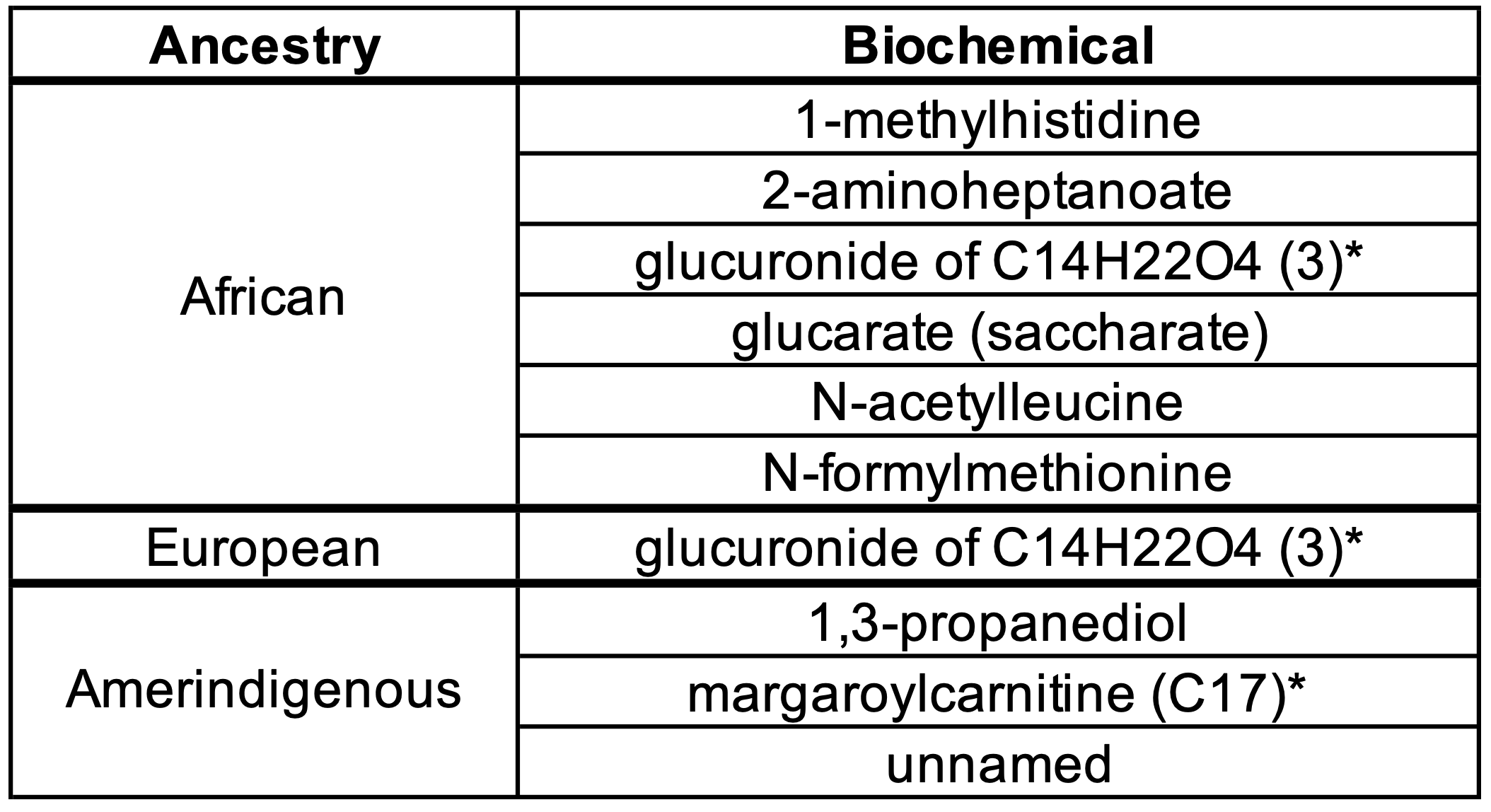
Results: Among all infants combined, we identified 130, 72, and 58 metabolites that associated with proportions of African, European, and Amerindigenous ancestry at p< 0.05, respectively, at timepoint 1, and 64, 33, and 39 metabolites, respectively, at timepoint 2. Ten metabolites were associated with the same ancestry, and in the same direction at both timepoints (Table 1). In analyses separated by race/ethnicity, 16 metabolites were associated with African ancestry, 5 with European ancestry, and 10 with Amerindigenous ancestry at p< 0.05; 22 of these were associated in the same direction with the same genetic ancestry in >1 racial/ethnic group (Table 2). Among metabolites that met significance criteria, 8 were involved in metabolic pathways, 2 were xenobiotics, 6 were unknown or partially characterize metabolites, and 6 were involved in other pathways.
Conclusion(s): We identified urinary metabolites whose levels vary with proportions of continental African, European, and Amerindigeous genetic ancestry among extremely premature infants. Our study shows that genetic ancestry impacts the urinary metabolome of extremely premature infants, and highlights the importance of considering genetic ancestry in metabolomic studies involving genetically diverse populations.
.png)