Neonatal Clinical Trials
Neonatal Clinical Trials 1
241 - Preterm Infants Receiving Osteopathic Manipulative Medicine Have Lower Risk of Neuromotor Developmental Delays and Decreased Length of Stay
Publication Number: 241.127

Angela K. Tyson, DO (she/her/hers)
Assistant Professor of Pediatrics, Neonatology Division
Golisano Children's Hospital at The University of Rochester Medical Center
Pittsford, New York, United States
Presenting Author(s)
Background: Osteopathic Manipulative Medicine (OMM) is a drug-free manual treatment that gently manipulates the body. International literature has shown improved outcomes and shorter hospitalization in preterm infants who receive OMM. After discharge, neurodevelopmental and motor delays contribute to ongoing morbidity for this patient group.
Objective: To determine if infants born < 34 weeks who receive OMM, compared to those who receive standard of care (SOC), will have lower risk for neuromotor delay as measured by the test of infant motor performance (TIMP) and decreased length of stay (LOS).
Design/Methods:
This was a single center, blinded randomized study comparing OMM and SOC in infants < 34 weeks gestational age (GA).
Intervention: Subjects < 28 weeks GA at birth received standard NICU care until 28 weeks corrected GA (cGA). Once >28 weeks cGA and study criteria met, randomization occurred 1:1 in 4 gestational age strata. Infants in the OMM (treatment) group then received weekly OMM until 36 weeks’ cGA; the control group received only SOC throughout. All parents and clinical care team members were blinded to group assignment, with exception of the primary investigator who performed OMM. At 36-37 weeks CGA, the TIMP was performed by a certified occupational therapist blinded to group assignment. The primary investigator was blinded from TIMP scores until the end of the study.
Analysis: TIMP scores and LOS were compared between groups using t test and Wilcoxon Rank Sum test; multiple linear regression was used to evaluate the treatment effect after controlling for covariates.
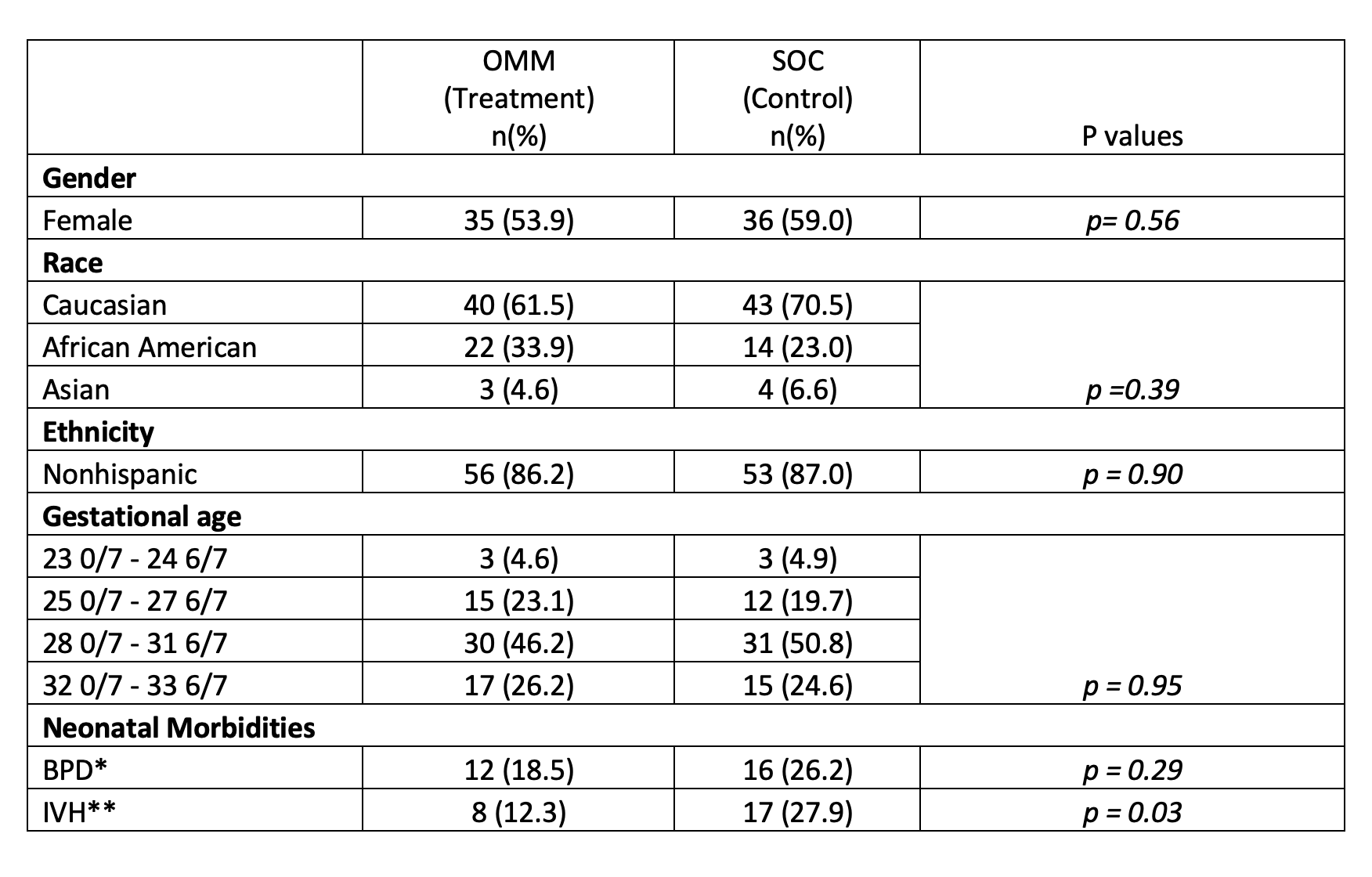
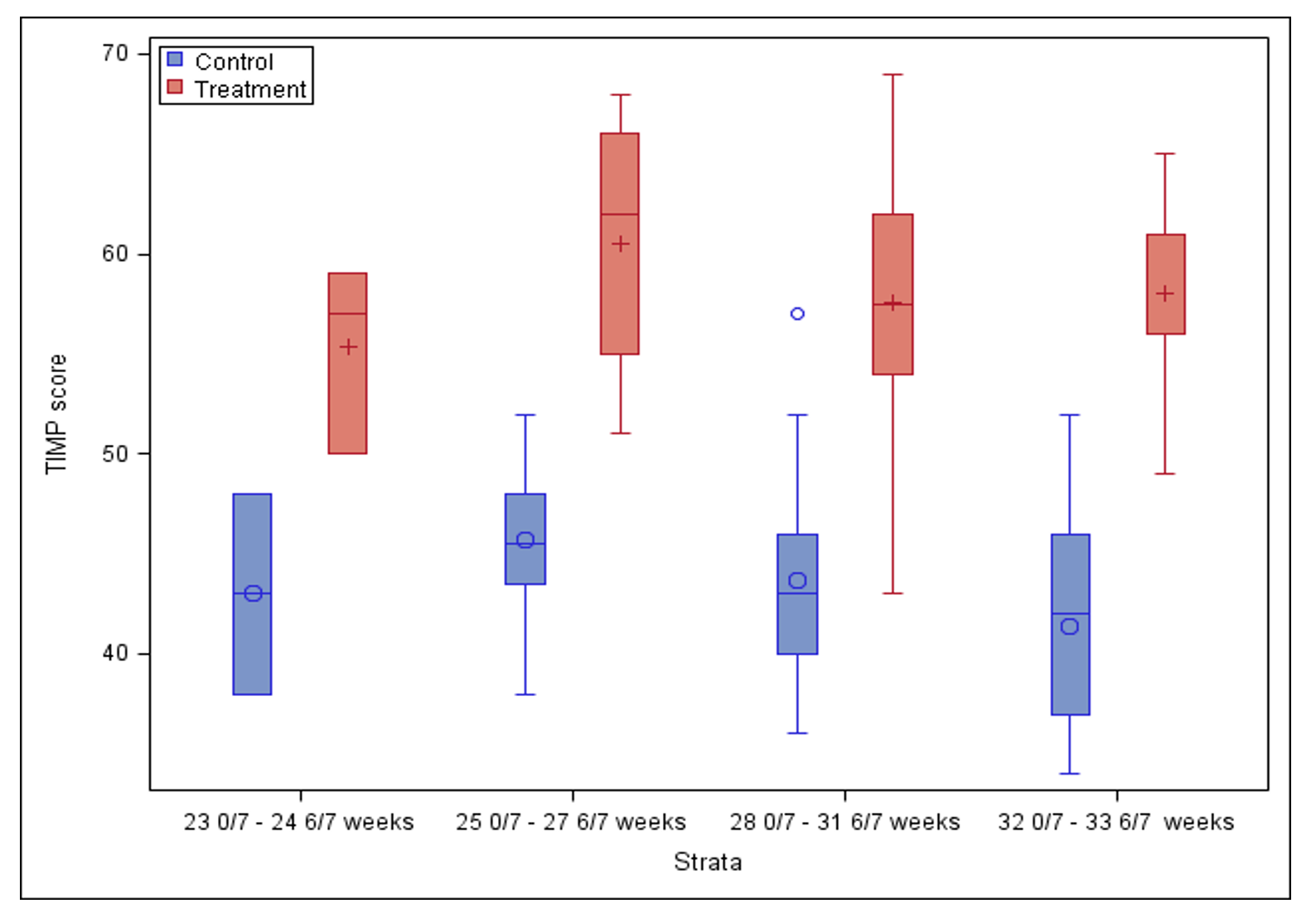
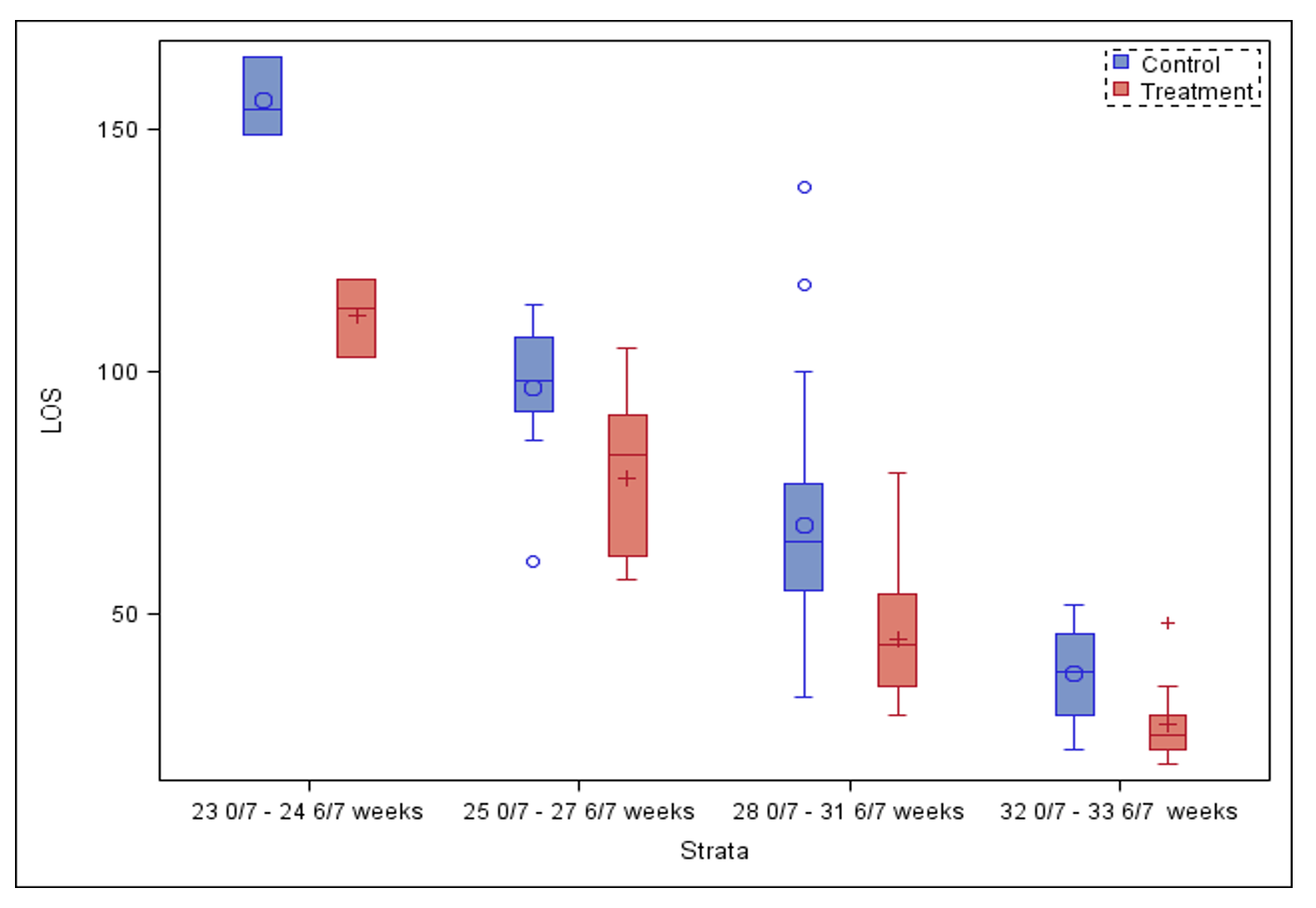
Results: There were 61 control and 65 treated subjects. Demographics are shown in Table 1. No baseline differences were detected between groups except for an increased incidence of grade I or II intraventricular hemorrhage (IVH) in controls (27.9% vs 12.3%, p=0.03). Treatment was associated with a higher TIMP score compared to control group, after adjusting for GA, sex, race, BPD and IVH (mean difference: 14.6; 95% CI: 12.7 – 16.2; p< 0.01) (Figure 1) and a shorter LOS (mean difference: 18 days; 95% CI: 12 – 23; p< 0.01) (Figure 2).
Conclusion(s): Preterm infants treated with OMM had meaningful reductions in length of stay and lower predicted risk of neuromotor developmental delays, as measured by the higher TIMP scores. Additional developmental screening will be performed at 1 and 2 years of age to assess persistence of the effect.