Infectious Diseases
Infectious Diseases 3
405 - Biodistribution of mRNA COVID-19 vaccines in human breast milk
Publication Number: 405.417
- NH
Nazeeh N. Hanna, MD (he/him/his)
NYU Langone-Long Island
Mineola, New York, United States
Presenting Author(s)
Background:
Most published clinical trials for the COVID-19 mRNA vaccines excluded pregnant and lactating women. Nevertheless, because of the mRNA vaccines' documented efficacy and safety profile, vaccination is recommended for breastfeeding women. However, there are very few published studies detailing the biodistribution of the mRNA vaccine, especially in lactating women. It is unknown if lactating infants are exposed to the mRNA vaccine after maternal vaccination. The CDC does not recommend COVID vaccination in infants < 6 months of age.
Objective:
To evaluate the presence of COVID-19 vaccine mRNA in breast milk (BM) after maternal vaccination and, if so, whether the mRNA in the BM is intact and translationally active.
Design/Methods:
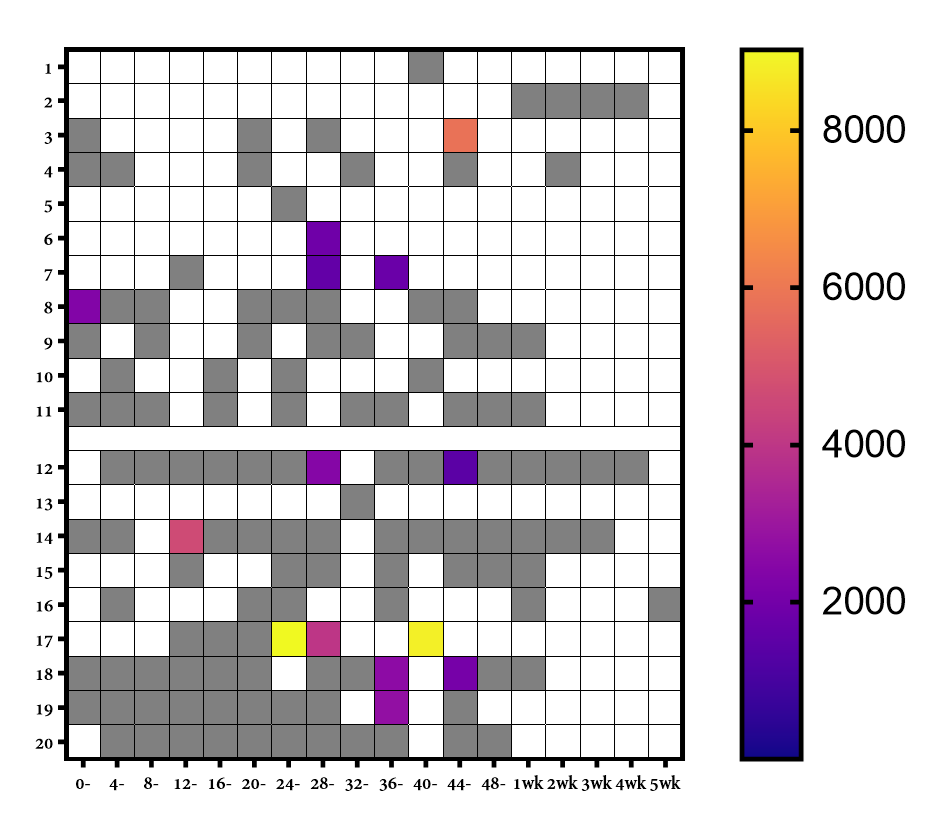
BM samples were self-collected from 20 lactating, healthy, post-partum women before receiving a COVID-19 mRNA vaccine and thereafter for up to 4 weeks post-vaccination. The presence of vaccine mRNA in whole BM and BM extracellular vesicles (EVs) was assayed by ddPCR. To investigate the integrity of vaccine mRNA in BM, linkage ddPCR was performed. The ability of the BM vaccine mRNA to induce SARS-CoV-2 spike protein expression was assessed after co-culture with the intestinal HT29 cell line.
Results:
Of 20 lactating women, trace amounts of COVID-19 mRNA vaccines were detected in 9 patients at various time points only in the first 48 hours post-vaccination. The vaccine mRNA appears to be present mainly in EVs. The BM vaccine mRNA maintained 12-25% integrity relative to the original mRNA vaccine. Furthermore, the EVs positive for mRNA content failed to induce spike protein in the HT29 cell line.
Conclusion(s):
This study report for the first time that the mRNA vaccine can spread systemically to be packaged into BM EVs, protecting it from degradation, including in the GI tract. Based on the sporadic presence, the trace quantities, and the absence of translational activity of the BM mRNA vaccine, we believe that breastfeeding after mRNA vaccination is safe, especially 48 hours post-vaccination. However, variable storage conditions after self-collected BM in our study may have led to mRNA degradation and potential underestimation of the vaccine mRNA BM content. Of note, the minimum mRNA dose needed to elicit an immune reaction in infants < 6 months of age is unknown. We believe transparency is warranted, and a dialogue between breastfeeding mothers and their clinicians regarding the benefit/risk considerations of breastfeeding in the first 48 hours after vaccination is needed. Future research should explore tissue biodistribution and pharmacokinetics of mRNA vaccines in lactating women.