Quality Improvement/Patient Safety: Primary & Subspecialty Outpatient Quality Improvement
QI 6: Primary & Subspecialty Outpatient QI Group 1
259 - Do They Really Have A Drug Allergy? General Pediatricians Collaborate with Allergists to Delabel False Penicillin Allergies
Publication Number: 259.452
- KS
Kristen Samaddar, MD (she/her/hers)
Associate Program Director, Clinical Associate Professor
Phoenix Children's Hospital
Phoenix, Arizona, United States
Presenting Author(s)
Background:
Approximately 10% of US patients report an allergy to penicillin-class antibiotics, making it one of the most reported drug allergies. Of those, 90% can tolerate penicillin (PCN) upon confirmatory testing. The prevalence of these false allergy labels leads to negative health outcomes and higher costs from the use of alternative antibiotics that are often less effective and lead to higher antibiotic resistance. Studies have shown PCN allergy labels are associated with increased rates of infection with MRSA, vancomycin-resistant enterococcus, C. Difficile and surgical site infections.
Objective:
< !1. Determine the baseline PCN allergy rate for Phoenix Children’s (PC) Primary Care, Complex Care, Adolescent Medicine (PCCCAM) patients
2. Refer >30% of patients with a PCN allergy label to PC Allergy and sustain by 7/1/23
3. Develop a system to track the number of patients delabeled
Design/Methods:
This quality improvement (QI) project used several interventions to achieve our aims: physician training, improved workflows, patient education materials, and increased collaboration between teams. We created and implemented a workflow for immediate delabeling if no risk of true allergy and referrals to allergists for at risk patients. Patients with PCN allergy labels, new allergy referrals, and scheduled or completed allergy appointments (process measures) were tracked monthly. We will be tracking the number of unique patients delabeled in PCCCAM or PC Allergy (outcome measure).
Results:
Pre-interventions, 386/5662 (7%) of patients at PCCAM had a PCN allergy label, 51/292 (17.5%) of patient with a PCN allergy label were referred to an allergist, and 27/292 (9.25%) had an appointment scheduled with PC Allergy. Post interventions (1/1-12/31/22), 241/415 (58%) of unique patients with a PCN allergy had an allergy referral, and 107/415 (26%) of unique patients with a PCN allergy had an allergy visit.
Conclusion(s):
Despite pandemic related staffing issues, we raised awareness for the importance of accurately assessing drug allergies. We enhanced communication between teams and streamlined the referral process based on families' input. Oral drug challenges are now offered the same day as initial consult. We have achieved the aim of referring >30% of patients with PCN allergies to allergists for oral challenges. Next steps include ongoing education for providers to ensure sustainability, optimizing allergists’ post-challenge communication, and improving tracking of patient who have been delabeled. By removing false allergy labels, we can impact healthcare for both the individual and system at large. 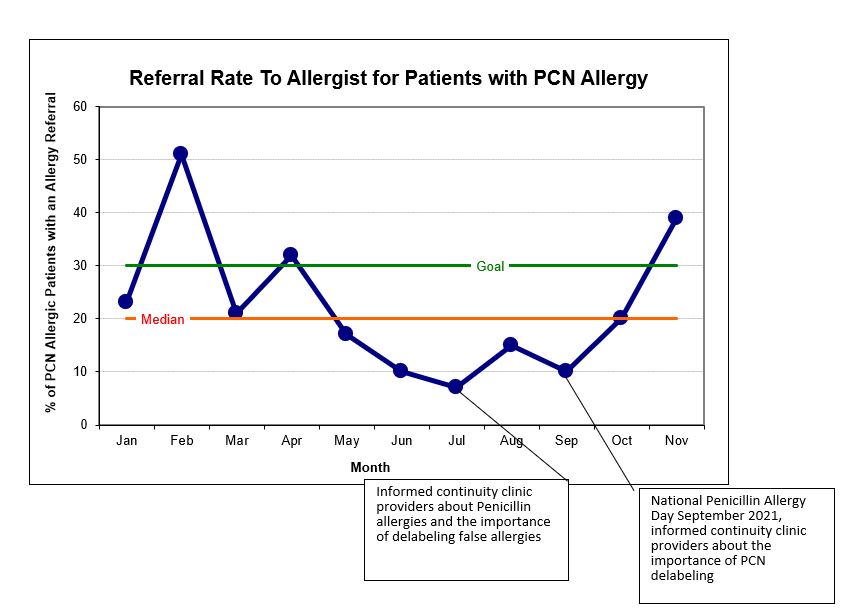
.jpg)
