Neonatal Neurology: Pre-Clinical Research
Neonatal Neurology 7: Preclinical 1
9 - MicroRNA-342 Increases Neuronal Cell Survival and Argonaute Protein Expression After Hypoxic-Ischemic Injury
Monday, May 1, 2023
9:30 AM - 11:30 AM ET
Poster Number: 9
Publication Number: 9.433
Publication Number: 9.433
Namood-e Sahar, University of Nebraska Medical Center, Omaha, NE, United States; Joslynn Hoburg, UNMC, Omaha, NE, United States; Eric S. Peeples, University of Nebraska Medical Center, Omaha, NE, United States

Namood-e Sahar, PhD (she/her/hers)
PostDoc
University of Nebraska Medical Center
Omaha, Nebraska, United States
Presenting Author(s)
Background: Neonatal hypoxic-ischemic encephalopathy (HIE) is a serious brain injury with minimal therapeutic options that can result in lifelong developmental impairment. A novel neuroprotective approach could involve the manipulation of microRNAs that are altered after HIE. Our previous studies have demonstrated several microRNAs (miRNA) that were increased or decreased in the brain after HIE. In order to alter messenger RNA expression, miRNAs rely on the RNA-Induced Silencing Complex made up of several proteins including Argonaute 2 (Ago2).
Objective: To evaluate the effects on cell survival and mechanism of action of altering miRNA expression in a cell culture oxygen-glucose deprivation (OGD) model of hypoxia-ischemia.
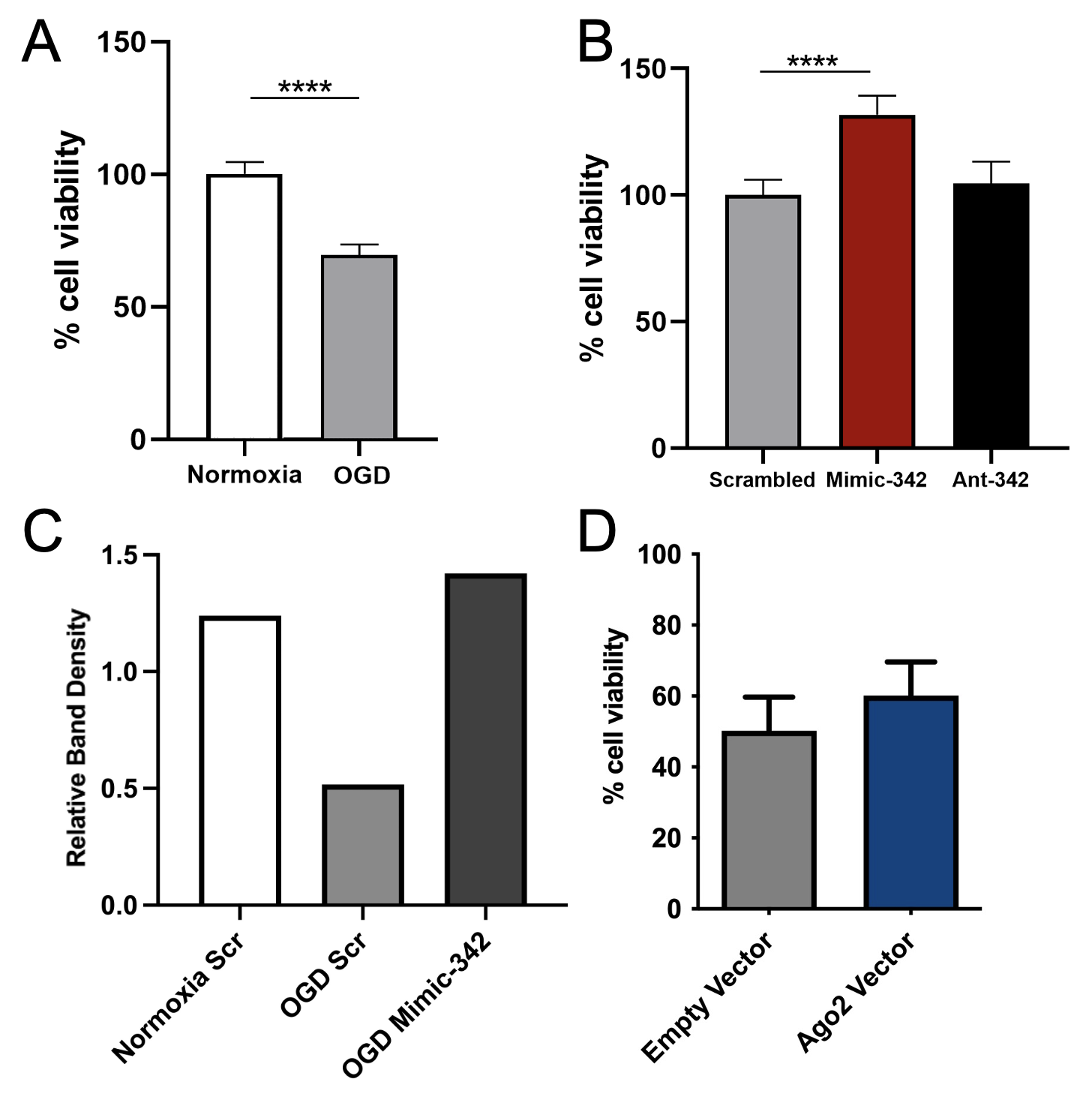
Design/Methods: The mouse neuroblastoma cell line Neuro-2a (N2a) underwent OGD by incubation for 6h in glucose-free medium and 1% O2 followed by replacement of glucose in the media and resumption of normoxia for reperfusion. Normoxia control cells remained in glucose-containing medium without oxygen manipulation. Cholesterol modified miRNA mimic and antagomir oligonucleotides were designed for miR-155, -335, -342, and -2137 based on endogenous expression seen in our previous mouse brain studies. A scrambled oligonucleotide was used as control. Oligonucleotides were added immediately after OGD and alamarBlue assay was used to analyze cell viability after 40h of reperfusion. As part of an immunoprecipitation experiment, it was noted that OGD decreased Ago2 expression, so cells were also collected for Ago2 expression as measured by western blot. Ago2 vector was administered 44h prior to OGD to increase Ago2 expression.
Results: OGD resulted in a 31% decrease in N2a cell viability compared to normoxia controls (Fig 1A). MiR-155, -335, and -2137 administration resulted in no change in cell survival compared to scrambled control. Administration of miR-342 mimic resulted in a 24% increase in cell survival of OGD treated cells (Fig 1B, p< 0.0001) as compared to scrambled control. OGD resulted in decreased Ago2 expression which was reversed by miR-342 mimic (Fig 1C). Increasing Ago2 alone prior to OGD did not significantly improve cell viability after OGD compared to empty vector (Fig 1D).
Conclusion(s): Increasing miR-342 expression resulted in improved cell survival when administered immediately after OGD injury in N2a cells. Ago2 protein expression was decreased after OGD, but similar to normoxia levels after miR-342 expression. Although increased Ago2 alone does not appear to protect against OGD-induced cell death, normalizing its expression may improve effectiveness of exogenous miRNA interventions.