Nephrology: CKD
Nephrology 5: CKD/ Diversity and Equity in Kidney Health
54 - Illuminating the Pediatric Bone Mineralization Defect in Clinical Practice
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 54
Publication Number: 54.35
Publication Number: 54.35
Marciana Laster, UCLA Mattel Childrens Hospital, Santa Clarita, CA, United States; Mark R.. Hanudel, University of California, Los Angeles David Geffen School of Medicine, Los Angeles, CA, United States; Isidro Salusky, University of California, Los Angeles David Geffen School of Medicine, Los Angeles, CA, United States

Marciana Laster, MD, MS
Assistant Professor of Pediatrics
UCLA Mattel Childrens Hospital
Santa Clarita, California, United States
Presenting Author(s)
Background: Unlike adults, defects in bone mineralization are highly prevalent in pediatric patients with CKD (both pre-dialysis and on dialysis). Presently, bone biopsy is the gold standard for diagnosis of the mineralization defect. Unfortunately, training in the use of pediatric bone biopsy in CKD remains limited. Therefore, the use of routinely available bone markers to predict abnormal mineralization is key.
Objective: The objective of this study was to build a predictive model of abnormal bone mineralization using circulating bone biomarkers.
Design/Methods: 87 pediatric patients with CKD (34 pre-D and 53 dialysis) underwent iliac crest bone biopsy and measurement of PTH, AP, phosphate, calcium, 25OHD, intact and c-terminal FGF23, sclerostin and Trap5B at the time of bone biopsy. CART modeling was performed to assess the prognostic ability of these biomarkers in discriminating abnormal bone mineralization. Performance of these models were summarized using the ROC curve (AUC) and AUC values were compared between models and PTH alone using the De Long’s test.
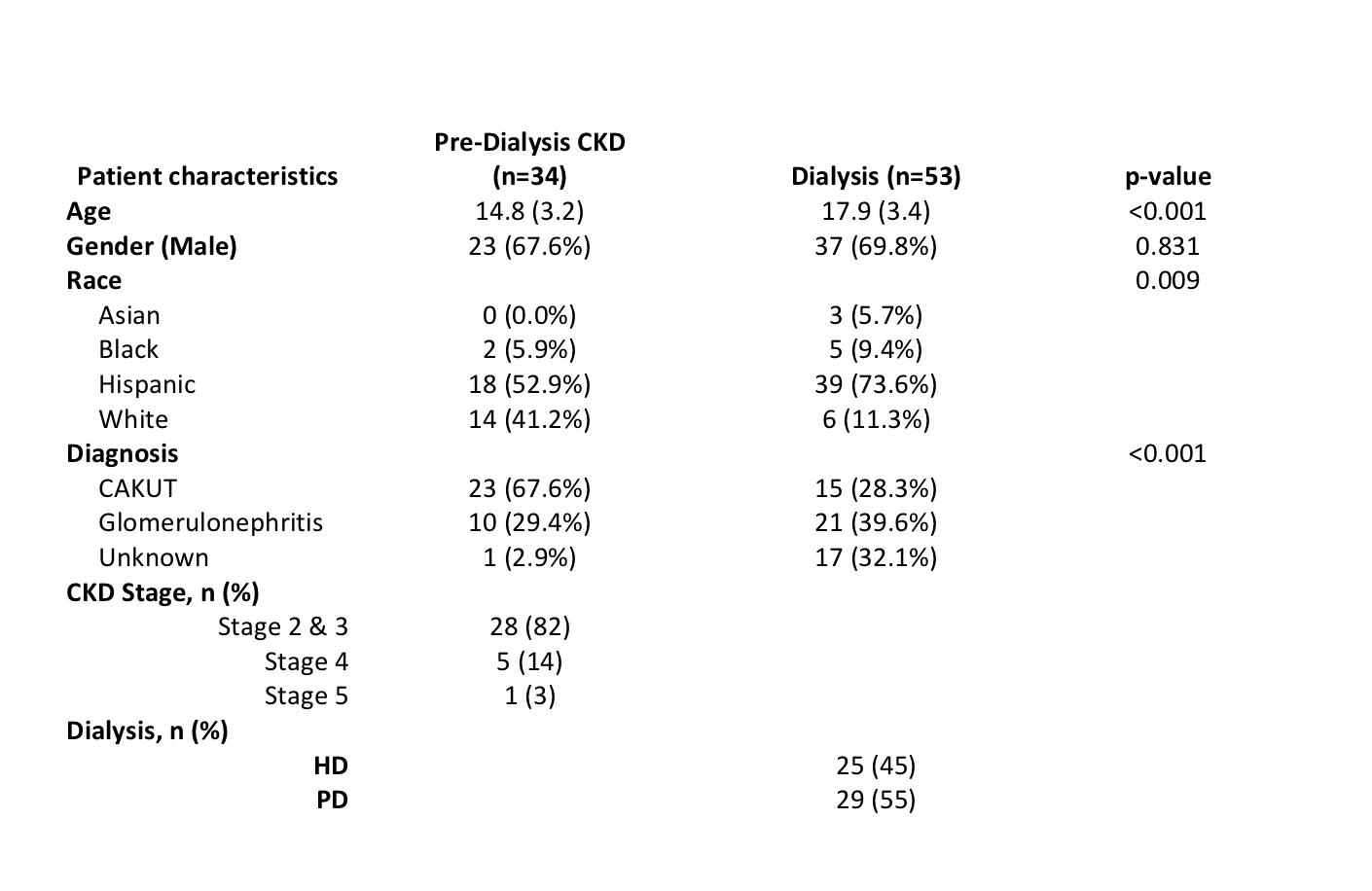
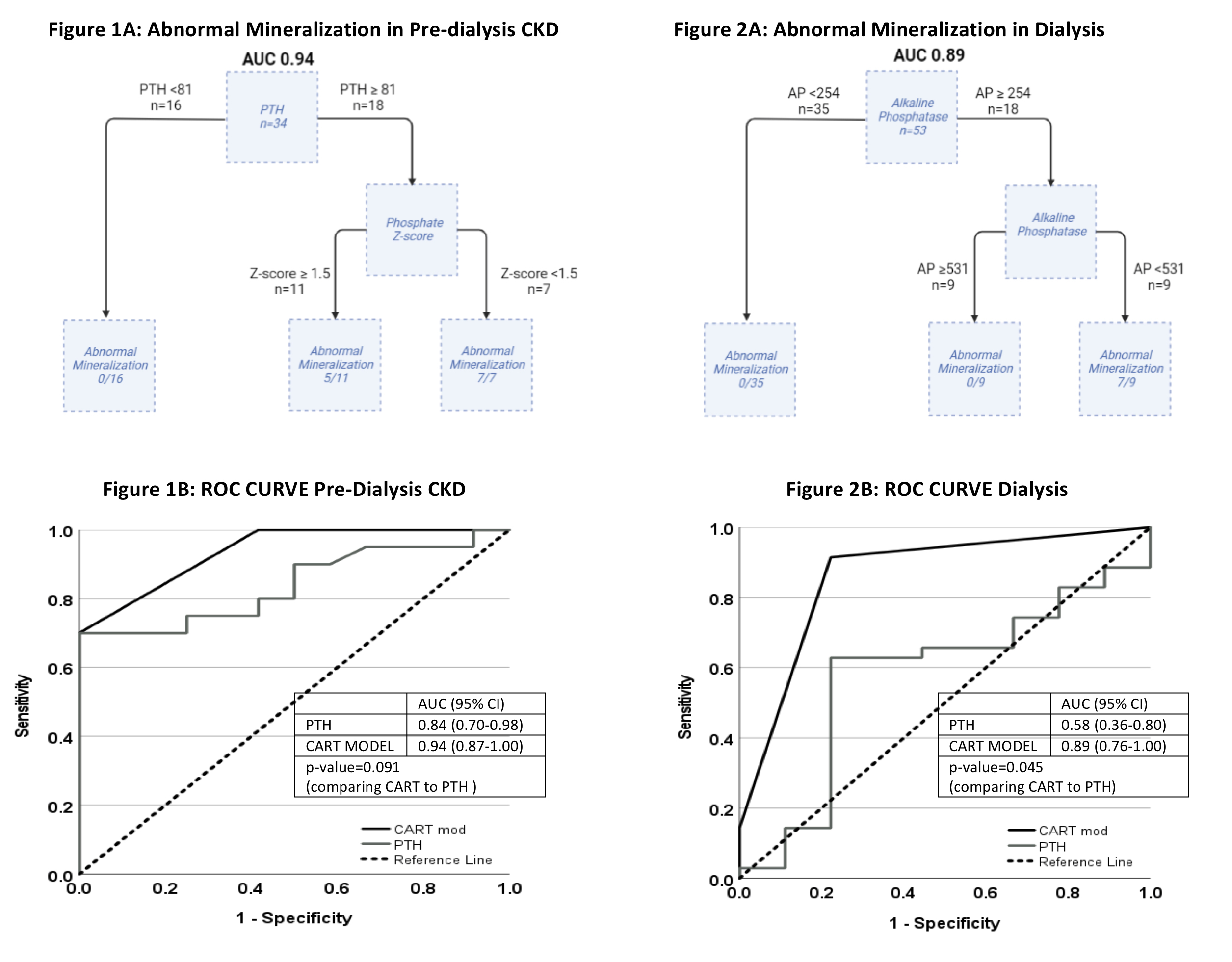
Results: Cohort characteristics are shown in Figure 1. In the pre-D CKD group, the combination of high PTH (≥81) and low phosphate z-score (< 1.5) was associated with abnormal mineralization [AUC 0.94 95%CI (0.87-1.00)] (Figure 1A). When compared to PTH alone, this model was not significantly different than PTH at predicting abnormal mineralization [AUC of PTH alone 0.84 (0.70-0.98), De Long’s p=0.091] (Fig 1B). In the CKD5D group, elevated alkaline phosphatase between 254 to 530 was associated with abnormal mineralization [AUC 0.89 (0.76-1.00)] (Fig 2A). When compared to PTH alone, AP was significantly better at predicting abnormal mineralization [AUC for PTH 0.58(0.36-0.80), De Long p=0.045) (Fig 2B). All other markers were not predictive of bone mineralization.
Conclusion(s): Routinely obtained biomarkers, AP and PTH, are useful in determining the presence of a mineralization defect in pediatric CKD. With further investigation, these findings may be directly applicable to the clinical management of pediatric patients with CKD.