Nephrology: Clinical
Nephrology 5: CKD/ Diversity and Equity in Kidney Health
51 - Kidney Effects of Sodium-glucose Cotransporter-2 Inhibitors in a Pediatric Population
Publication Number: 51.35

Claire B. Sandler, B.S. (she/her/hers)
Clinical Research Coordinator
Ann & Robert H. Lurie Children's Hospital of Chicago
Chicago, Illinois, United States
Presenting Author(s)
Background:
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) have transformed care for adult patients with type II diabetes (T2D), heart failure (HF), and chronic kidney disease (CKD). In adult trials, most participants experience an acute drop in glomerular filtration rate (GFR) after starting SGLT2i. Despite this, use of SGLT2i is strongly associated with improved long-term kidney outcomes.
Objective: There is a paucity of data describing the use and impact of SGLT2i in pediatric patients, especially with regards to kidney function and disease. The objective of this study was to observe the short-term effects of SGLT2i on kidney function of pediatric patients.
Design/Methods:
We performed a retrospective study of patients aged 0-25 years who were prescribed Empagliflozin or Dapagliflozin at our institution between December 2021 and December 2022. Data was collected from patient medical records. Laboratory data, including serum creatinine at baseline, 2-week, 1-month, and 3-month intervals, were collected. We used the CKiD Under 25 formula (creatinine only) to estimate GFR. Our primary outcome was the change in eGFR from baseline to 3-months after starting the medication.
Results:
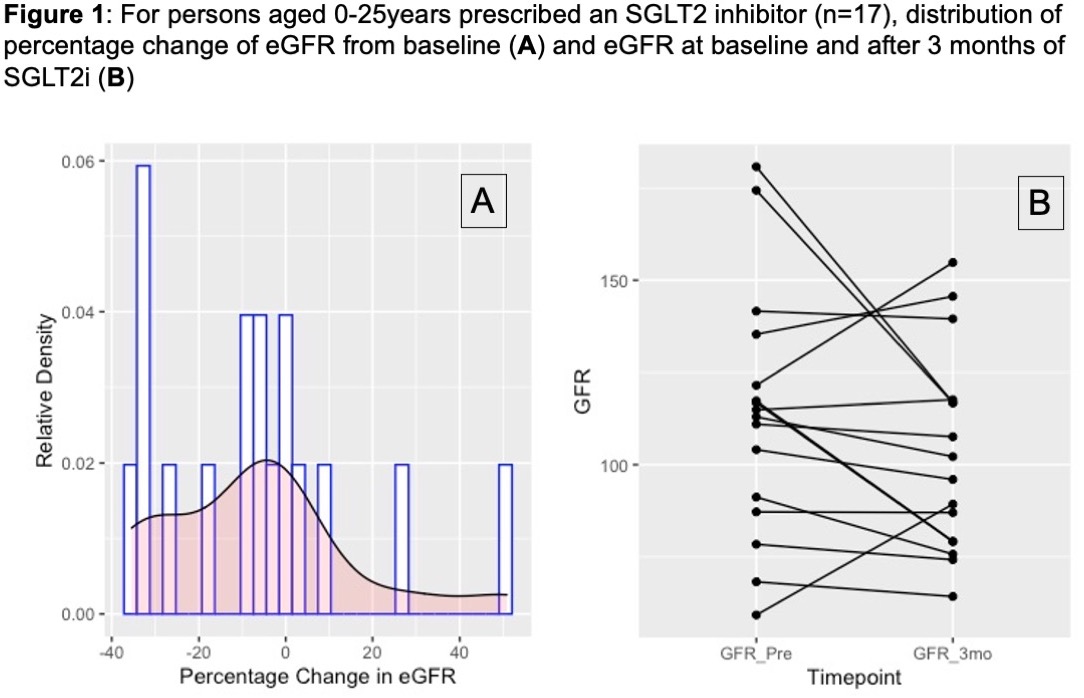
Of the 42 patients prescribed an SGLT2i in the past year, 17 patients had available lab data to be included in our analysis. Baseline diagnosis was HF in 53% (n= 9), Glycogen Storage Disease Type 1B in 29% (n=5), and T2D in 24% (n=4) of participants. One patient was diagnosed with CKD and received a kidney transplant 2 years prior to beginning Empagliflozin. Mean (±Standard Deviation) baseline age was 17 (±5.6) years and eGFR was 121 (±46) mL/min/1.73m2. With this population in mind, 24% (n= 4) of patients experienced an increase in GFR, while 76% (n= 13) of patients experienced a decline in GFR between their baseline and 3-month interval measurements. In total, 59% (n= 10) experienced a decline in GFR greater than 5%, and 41% (n= 7) experienced a decline in GFR greater than 10% (Figure 1).
Conclusion(s):
Among pediatric patients at our center who received an SGLT2i prescription in the past year, most patients experienced an acute decline in GFR within 3-month follow up kidney function panels. This data suggests SGLT2is may have similar clinical effects in youth to what was observed in large trials in adults. We are undertaking future analyses to study changes in blood pressure and hemoglobin over the same interval, along with safety and tolerability assessments.