Nephrology: Transplant
Nephrology 4: Transplant
34 - New onset diabetes after transplant in a single pediatric kidney transplant center
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 34
Publication Number: 34.349
Publication Number: 34.349
Anjali Khanna, Emory University School of Medicine, Decatur, GA, United States; Rouba Garro, Emory University School of Medicine, 2015 uppergate Dr NE, GA, United States; J. Nina Ham, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States; Margret Kamel, Emory University School of Medicine, Atlanta, GA, United States; Rochelle Liverman, Children's Healthcare of Atlanta, Atlanta, GA, United States

Anjali Khanna, MBBS, MPH (she/her/hers)
Clinical research coordinator
Emory University School of Medicine
Decatur, Georgia, United States
Presenting Author(s)
Background: The prevalence of new-onset diabetes after transplant (NODAT) in pediatric kidney transplant (KT) recipients varies among studies due to the lack of a consistent definition. Risk factors and pathogenesis associated with its onset are poorly understood.
Objective: The objective of this study is to evaluate the prevalence of and risk factors for NODAT at a high-volume, tertiary-care pediatric hospital, and characterize the disease course in NODAT patients.
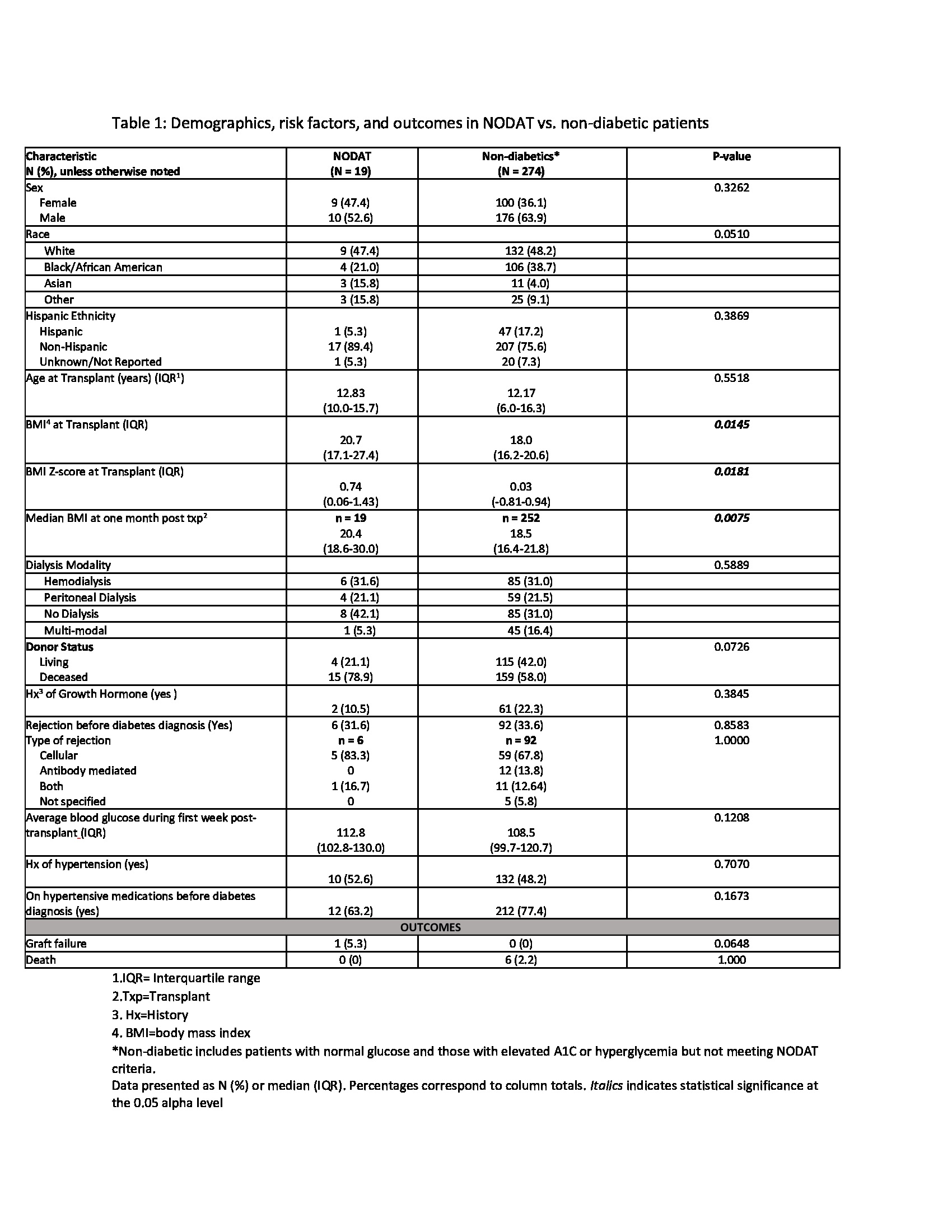
Design/Methods: We performed a retrospective review of pediatric KT recipients transplanted between 2006-2016 at our center. We excluded patients with multi-organ transplants and with pre-existing diabetes. We defined NODAT as persistent hyperglycemia with serum glucose >200 mg/dl, HbA1c >6.5%, and requiring antihyperglycemic medication for ≥ 30 days. We compared demographic and transplant characteristics between NODAT (n=19) and non-diabetic patients (n=274) using Chi-square tests and Wilcoxon rank sum tests. We performed a further evaluation of NODAT patients to evaluate risk factors and outcomes.
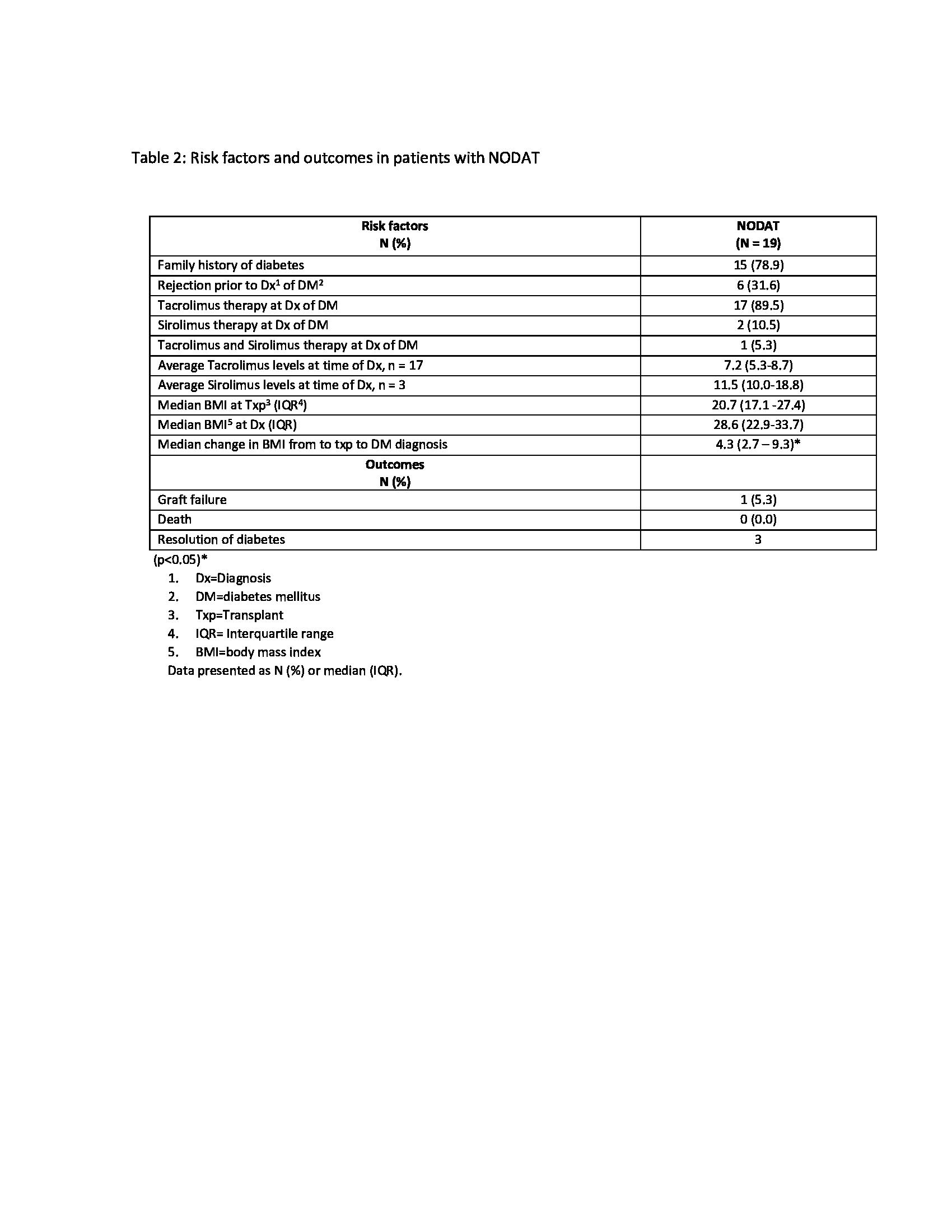
Results: Over the 10-year period, 293 patients met the inclusion criteria with the median age at transplant of 12.8 years. Nineteen patients (6.48%) developed NODAT, 29 patients had elevated HbA1c (5.7-6.4%), 43 patients had hyperglycemia (serum glucose levels 140-199 mg/dl), and 202 patients had normal serum glucose levels. Comparisons of patients with and without diabetes revealed that NODAT patients had higher BMI-Z scores at the time of transplant (p=0.02) (table 1). The median time from transplant to development of NODAT was 17 months (25th-75th: 4 – 83). Most patients who developed NODAT had a family history of diabetes in first-degree relatives (79%), received Deceased Donor KT (78.9%), were maintained on tacrolimus at the time of diagnosis (67%), and had a significant median change in BMI from time of transplant to DM diagnosis (p< 0.05) (Table 2). NODAT diagnosis and Insulin initiation occurred in the context of active rejection episodes in three patients. Despite a more stringent definition of NODAT, 2/19 (10%) patients came off insulin therapy and were normoglycemic at 1, 5, and 8 years post DM diagnosis.
Conclusion(s): A more consistent definition of NODAT and larger studies are warranted to better understand the prevalence, risk factors, and pathogenesis of hyperglycemia and diabetes post-transplant. Further evaluation of the effect of NODAT and glucose intolerance on graft function in this cohort of pediatric KT recipients is underway.