Neonatal/Infant Resuscitation
Neonatal/Infant Resuscitation 2
315 - The Air for Infant Resuscitation (AIR) Prospective Cohort Study: Moderate-late preterm infants do not achieve oxygen saturation targets during delivery room resuscitation
Publication Number: 315.348

James X. Sotiropoulos, BMed/BSc(Hons)/MD Student, MPhil Candidate (he/him/his)
Medical Student and MPhil Candidate
University of Sydney
Sydney, New South Wales, Australia
Presenting Author(s)
Background: In very preterm infants (< 29 weeks’ gestation), evidence suggests that failure to achieve oxygen saturations (SpO2) >80% by 5 minutes of life increases risk of death and/or disability. Expert guidelines recommend using low levels of fractional inspired oxygen (FiO2, 0.21-0.3) to initiate respiratory support for moderate to late preterm (MLPT, 32-36 weeks gestation) infants at birth to decrease oxidative stress but whether this strategy achieves target SpO2 is unknown.
Objective:
To determine if initiating respiratory support with FiO2 0.21-0.3 in MLPT infants achieves SpO2 targets recommended by clinical guidelines.
Design/Methods: This is a prospective, opportunistic, observational study with consent waiver. Preductal SpO2 readings were obtained from eligible infants (32-36 weeks’ gestation, need for respiratory support with positive pressure and/or ventilation) from 5 hospitals in New South Wales, Australia. SpO2 and heart rate (HR) were recorded via the Masimo ROOT system. Minutely FiO2 was recorded manually. Primary outcome was reaching a minimum SpO2 80% at 5 minutes. A total of 71 infants were needed to detect an absolute risk difference of 15% in study infants to reach SpO2 80% by 5 minutes compared to population norms (75% v 65%, alpha 0.05, power 0.8). The study was prospectively registered (ACTRN12620001252909).
Results:
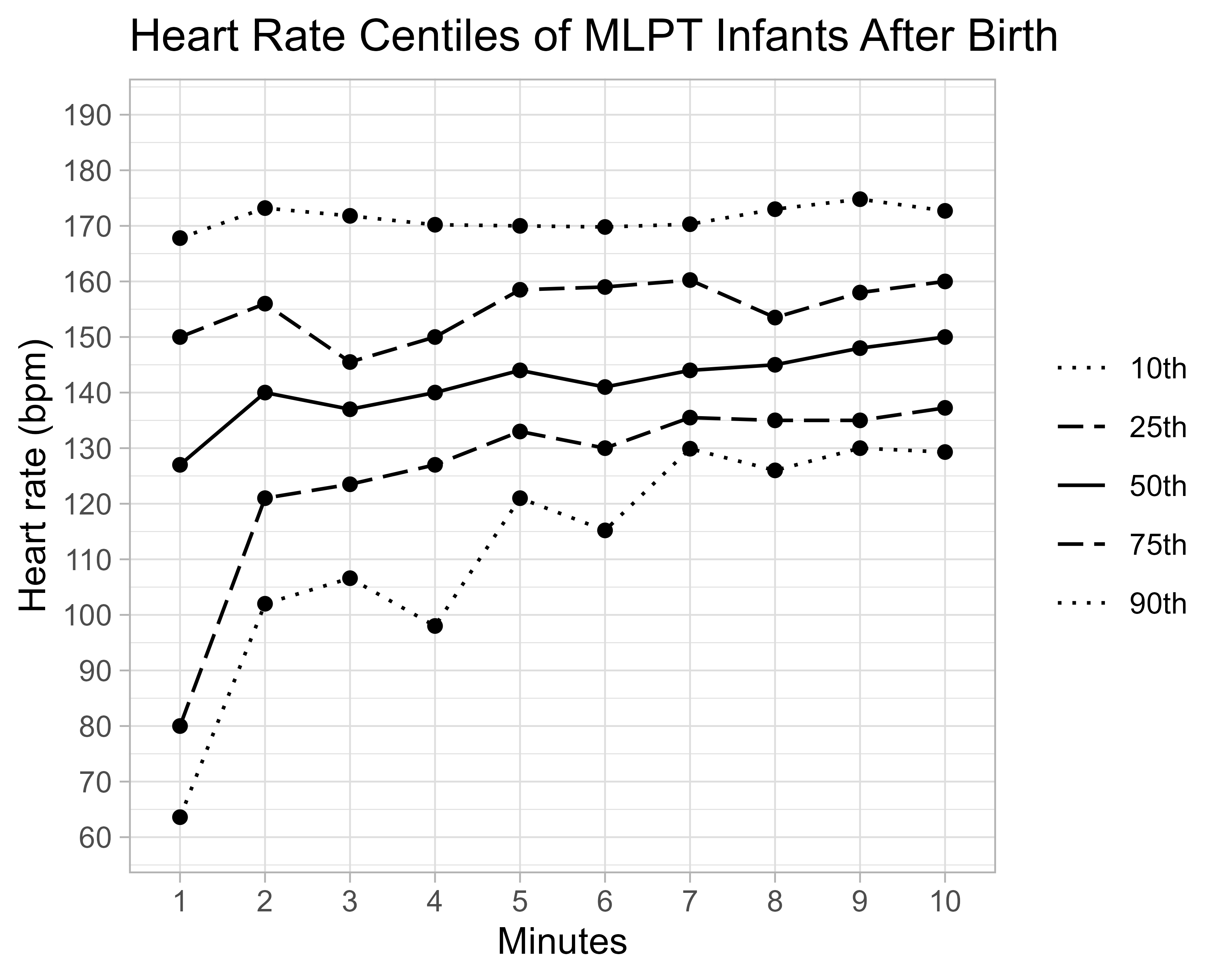
A total of 79 infants were recruited between March 2021 and Dec 2022 (Table 1). Most (n=55, 75%) had initial respiratory support with FiO2 0.21 (range 0.21-1.0) by CPAP (n=70, 96%). Five (7%) were intubated. Median SpO2 was 81% (IQR 67-90) at 5 min and 93% (IQR 86-96) at 10 minutes (Fig 1). A total of 25 (58%) infants did not reach SpO2 80% at 5 mins. Only 8 (19%) infants met 5 min ILCOR SpO2 targets of 80-85%. By 10 mins, median SpO2 in infants given FiO2 0.21 was lower than those given FiO2 >0.3 (0.21: 90%, >0.3: 96%, p=0.038). Median HR was similar between groups at five (144 bpm, IQR 133-158) and ten minutes (150 bpm, IQR 137-160) (Fig 2). No infant required cardiac massage or adrenaline.
Conclusion(s): Most MLPT infants requiring respiratory support have lower SpO2 than those recommended in clinical guidelines, especially if resuscitation is started with FiO2 0.21. The impact of this on critical health outcomes including neurodevelopment, death and other major morbidities are unknown. Further research with robust randomized controlled trials, powered to examine important clinical outcomes, is warranted to investigate the most appropriate delivery room oxygen supplementation practices for MLPT infants..png)
.png)
