Neonatal General
Neonatal General 9: NOWS and Other Exposures
196 - Initial Observations of an RCT Comparing NASSCORES to Finnegan Scores
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 196
Publication Number: 196.333
Publication Number: 196.333
Hannah Pee, Maria Fareri Children's Hospital, New York Medical College, New York, NY, United States; Manuel H. DeCastro, New York Medical College, Central Valley, NY, United States; Edmund F.. LaGamma, New York Medical College, Valhalla, NY, United States; Prabhakar Kocherlakota, New York Medical College, Newburgh, NY, United States
.jpg)
Hannah Pee, MD (she/her/hers)
Neonatal-Perinatal Medicine Fellow, PGY-6
Maria Fareri Children's Hospital, New York Medical College
New York, New York, United States
Presenting Author(s)
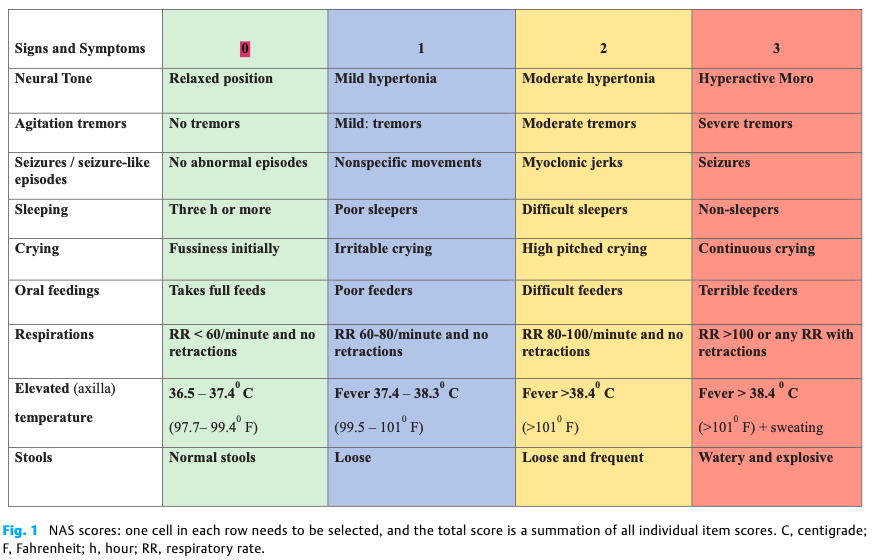
Background: The Modified Finnegan’s Neonatal Abstinence Scoring Tool (MFNAST) is widely used in most hospitals to diagnose and guide pharmacotherapy for Neonatal Abstinence Syndrome (NAS). Despite its widespread use, the MFNAST is long, complex, and has highly variable inter-rater reliability. Recent studies have showed that simplified scoring systems are equally sensitive and specific for the diagnosis of NAS. NASSCORES is a 9-item simplified score developed by Kocherlakota et al in 2016 that has good agreement and correlation with MFNAST, where NASSCORES of 4 and 6 were equivalent to MFNAST of 8 and 12 respectively.
Objective: To compare the safety and efficacy of NASSCORES against MFNAST in the management of neonatal abstinence syndrome.
Design/Methods: This multi-center open labelled 1:1 randomized study was initiated in multiple hospitals from Jan 2022. NAS symptoms were monitored either by NASSCORES or by MFNAST scores, which guided initiation, escalation and weaning of pharmacological treatment. Threshold scores were NASSCORES of 4 and MFNAST of 8. Nonpharmacological measures were continued as before during the period of study. Outcomes measured included length of stay and need for supplemental medications.
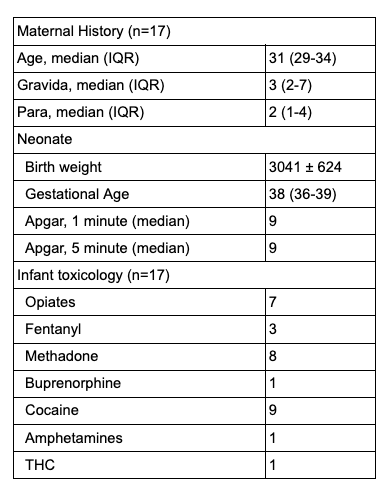
Results: 24 patients were enrolled in the study after parental consent was obtained. 7 infants were excluded from analysis due to prematurity, congenital malformations, and lack of need for pharmacological treatment. The mean length of methadone treatment was 14.2 ± 5.3 days in the NASSCORES group and 14.6 ± 8 days in the MFNAST group. No infants in the NASSCORES group required any supplemental medications while 2 infants in the MFNAST group received phenobarbital supplementation.
Conclusion(s): This is the first RCT comparing two scoring systems in the management of NAS. Infants managed using NASSCORES have similar length of pharmacological treatment with those managed with MFNAST, with the advantage of being easier to apply by nurses. More patients are needed in this study to further compare both scores.
.png)
