Immunizations/Delivery
Immunizations/Delivery 2
429 - Feasibility and acceptability of recommending HPV vaccine at ages 9-10 years in two states
Publication Number: 429.323

Caroline Tietbohl, PhD (she/her/hers)
Assistant Professor
University of Colorado School of Medicine
Aurora, Colorado, United States
Presenting Author(s)
Background:
Human papillomavirus (HPV) causes most cervical, anogenital and oropharyngeal cancers. An effective and safe vaccine exists but is vastly underutilized. HPV vaccine is routinely recommended at ages 11-12 but may be given as early as 9-10 years. Little data exist about feasibility or acceptability of initiating HPV vaccination prior to age 11.
Objective: To describe primary care provider and staff experiences with the feasibility and acceptability of initiating HPV vaccine at ages 9-10 years.
Design/Methods: This explanatory sequential mixed-methods study was undertaken as part of an ongoing randomized trial comparing initiation of HPV vaccine at age 9-10 years (intervention) versus 11-12 years (control) across 17 practices in CO (9 intervention) and 16 practices in CA (8 intervention). We administered a survey to intervention practice providers at 1-month and conducted semi-structured interviews with providers and staff at intervention practices at 3 months. We used descriptive statistics to analyze survey data and used content analysis to analyze interviews.
Results: A total of 66 (92%) CO providers and 39 (87%) CA providers completed the 1-month surveys. Interview participants included clinicians (n=18) and staff (n=17) from all CO and CA intervention practices. Of survey respondents, 59 (90%) in CO and 30 (77%) in CA were routinely recommending HPV vaccine at ages 9 or 10. Among these, most respondents reported that early recommendation was not burdensome and that select factors associated with HPV vaccine initiation were largely unchanged, including parental concerns, parental pushback, and vaccine discussion times (Figure 1). Interviews confirmed and expanded on survey results (Table 1). Interviewees reported that many parents were receptive to discussing HPV vaccination at ages 9-10 and that pushback often stemmed from child expectations for a vaccine-free visit at age 9 rather than concerns about the vaccine. Interviewees also reported that discussions at age 9-10 were shorter because sexual activity was not salient. Additionally, interviewees preferred having the option to space out vaccines because this allowed for more opportunities to discuss and complete the HPV vaccine series and because administering a single vaccine at age 9 (and fewer at age 11) may be more comfortable for children.
Conclusion(s): These findings show that recommending HPV vaccine at ages 9-10 was feasible for clinicians, compatible with workflows across various practices and generally acceptable to parents. Full study results will determine if initiating HPV vaccination earlier increases vaccination completion rates.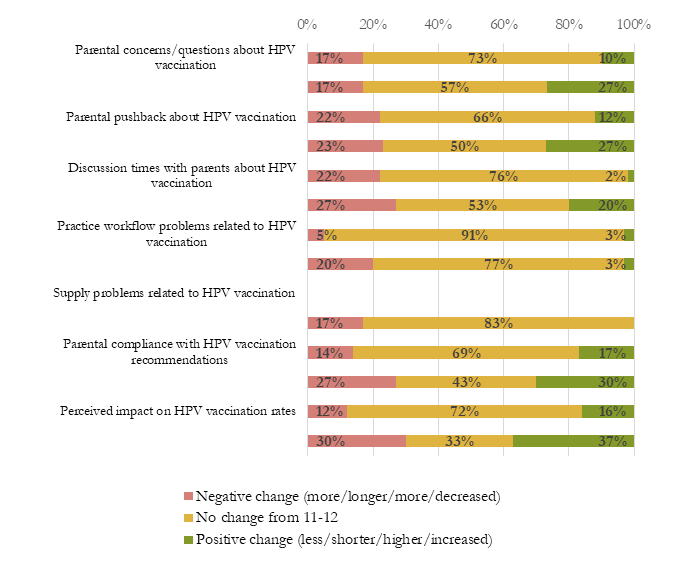
.png)
