Neonatal Hematology & Bilirubin Metabolism
Neonatal Hematology & Bilirubin Metabolism 1: Bilirubin
125 - Chemo-Prevention of Neonatal Exchange Transfusion: Compassionate Ongoing Trial of the Novel Nutraceutical Nanodrug
Publication Number: 125.238

Asim K. mallick, MD (he/him/his)
Professor
NRS Medical College and Hospital
Kolkata, West Bengal, India
Presenting Author(s)
Background: Citrate functionalized trimanganese tetroxide nanoparticles (C-Mn3O4 NPs) was reported for its chemo-preventive properties for acute bilirubin encephalopathy in a rodent model (Ped. Res. 10.1038/s41390-022-02179-5).
Objective: Following an institutional ethics review of the potential risks and benefits of this approved nutraceutical supplement, we planned a controlled open label compassionate trial in a small select cohort of neonates who were referred to our intensive care unit with imminent risk for acute bilirubin encephalopathy, in need of a neonatal exchange transfusion and being offered emergency use of phototherapy.
Design/Methods:
Nanodrug supplement (single oral dose: 0.25 mg/kg) was administered after informed parental consent as intensive phototherapy was administered while preparations were made to access blood and initiate the procedures based on indications and thresholds recommended by the 2004 AAP Guidelines. Vital signs were measured continuously along with serial TSB at 2, 6, 12, and then every 24 hours. Clinical follow for neonatal well-being and neurological examination were at ages 7 days and 4 weeks and scheduled for ages 3 to 6 months. We report our clinical and follow-up experiences for 29 term and late-preterm neonates who met eligibility criteria upon admission from October 1, 2022, through December 1, 2022.
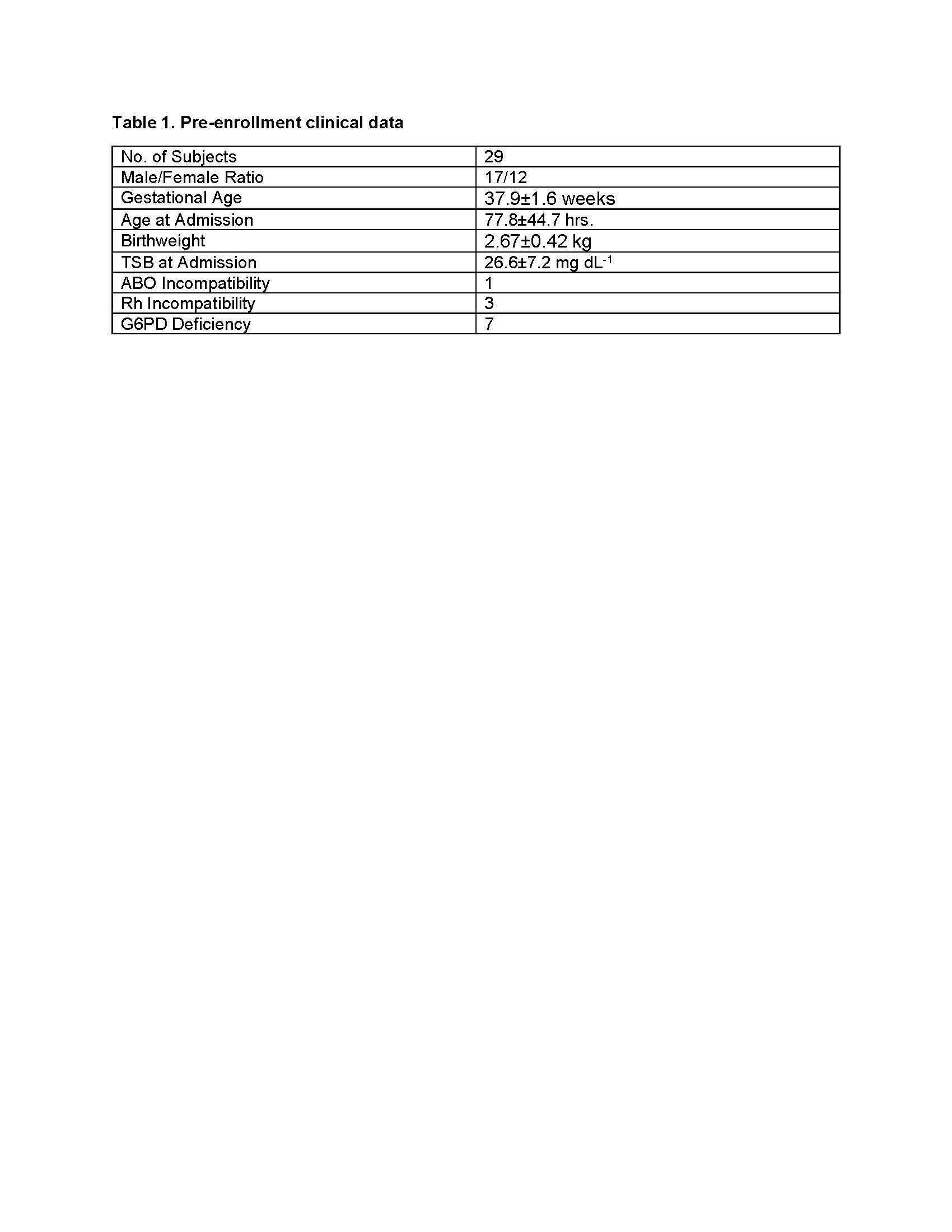
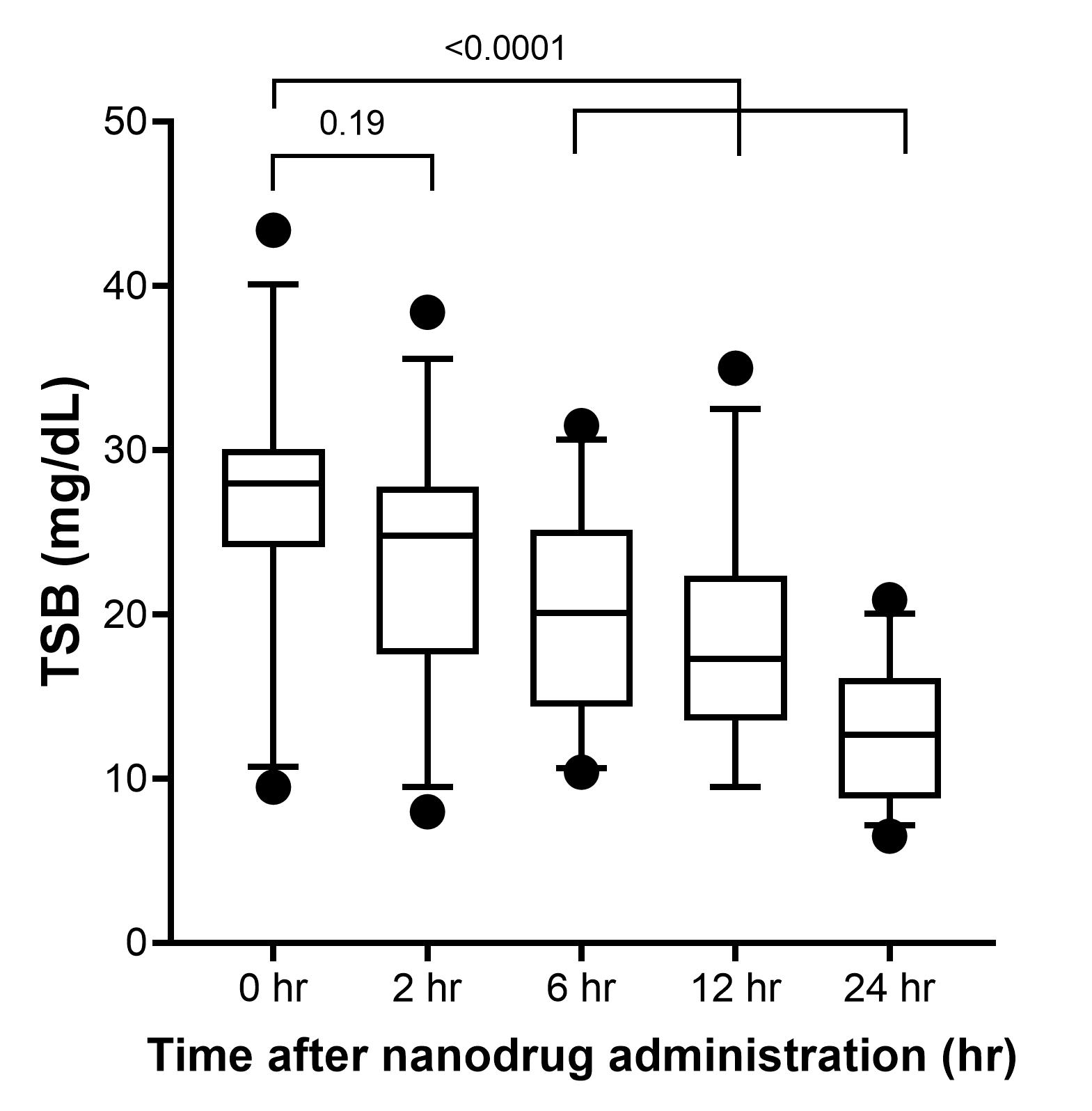
Results: Mean birthweight was 2.67±0.42 kg and GA of 37.9±1.6 weeks (range 35 to 42 weeks; 12/29 were females. Pre-enrollment clinical data is listed in Table 1. Exchange transfusion, a single double volume procedure, was only conducted in 5 /29 neonates with TSB >28 mg dL-1. TSB level dropped significantly (p< 0.001) within 4 hours of dose administration from 26.5±7.2 mg dL-1 to 19.9±6.1 mg dL-1) and followed by continued decline over the ensuing 24 hours to 10.5±3.4 mg dL-1). At the 7 day follow up TSB value were < 40th percentile for age in hours. At follow-up, none had post-icteric neurologic deficits or auditory impairment (by auditory evoked response testing). No clinical adverse events were noted during this early follow-up.
Conclusion(s):
We observed an effective and rapid decline in TSB values among neonates who were at imminent risk for exchange transfusion concurrent to use of phototherapy. Thus far, our pilot observations suggest that 24/29 neonates did not go onto need to undergo an exchange transfusion and none sustained neurologic deficits. Long-term safety has yet to be verified and a randomized multi-centered clinical trial should be considered.