Neonatal Hematology & Bilirubin Metabolism
Neonatal Hematology & Bilirubin Metabolism 1: Bilirubin
116 - Unbound Bilirubin and Risk of Severe Neurodevelopmental Impairment in Extremely Low Birthweight (ELBW) newborns
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 116
Publication Number: 116.238
Publication Number: 116.238
Cody Arnold, Stanford University School of Medicine, Oakland, CA, United States; Ivana Maric, Stanford School of Medicine, Stanford, CA, United States; Ronald J. Wong, Stanford University School of Medicine, Stanford, CA, United States; william oh, The Warren Alpert Medical School of Brown University, providence, RI, United States; Jon E.. Tyson, McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, United States; David Stevenson, Stanford University School of Medicine, Stanford, CA, United States

Cody Arnold, MD, MSc (he/him/his)
Associate Professor
Stanford University School of Medicine
Palo Alto, California, United States
Presenting Author(s)
Background: A single unbound bilirubin (UB) level was measured on day 5±1 in 1101 ELBW newborns enrolled in the Neonatal Research Network Randomized Controlled Trial (RCT) of Aggressive versus Conservative Phototherapy (PT) in ELBW Newborns. A secondary study reported a statistically significant association of UB with risks of death and death or neurodevelopmental impairment (NDI) but the UB exposure risk of NDI among survivors was not reported.
Objective: To quantify UB exposure risk of severe NDI for surviving ELBW newborns.
Design/Methods: UB was measured using a UB-A1 Analyzer (Arrows, Japan) and standardized between labs as percentiles of Z-scores. Severe NDI for this secondary study was defined at 18-22 months corrected age as a score of ≤50 on the Bayley II Mental or Psychomotor Developmental Index or gross motor function score of 5 (movement requires adult assistance) or severe bilateral hearing loss. UB exposure was analyzed as a binary variable given a clear cut-point for severe NDI. The independent effect of UB was estimated using ensemble learning targeted maximum likelihood estimation (ELTMLE, https://github.com/migariane/eltmle) and standard logistic regression (LR), adjusting for gestational age, SGA, sex, multiple birth, antenatal steroids, outborn, severe IVH, maternal education, insurance type, clinical stability at blood sampling, and perinatal center.
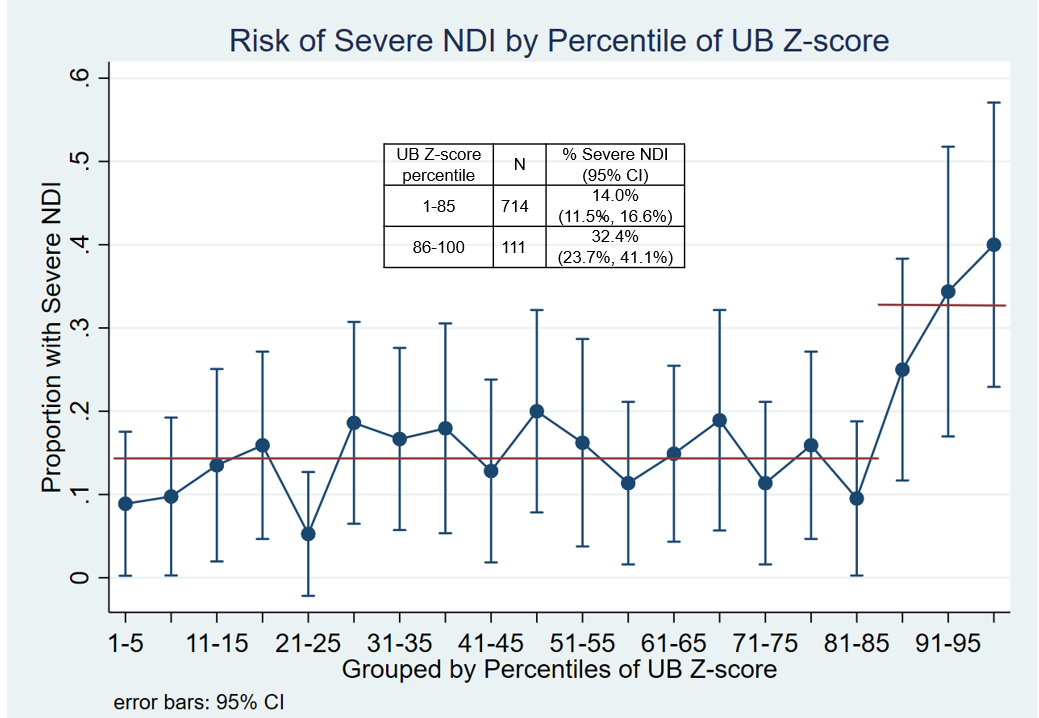
Results: 825 infants survived and completed evaluations allowing assignment of NDI status. The correlation of UB with total serum bilirubin (TSB) measured in the same blood sample was poor: R2=0.24. There was a sharp increase in risk of severe NDI at the 85th percentile of the UB Z-scores (Figure). The point estimates for >85th increased risk were similar with ELTMLE (13.3%) and LR (12.5%) but the 95% confidence intervals were narrower with ELTMLE: 9.3-17.3% vs 1.7-23.3% (Table 1). The increased risk associated with UB >85th was 2nd largest relative to known NDI risk factors in the LR model (Table 2). Among 415 ELBW newborns randomized to aggressive PT with TSB ≥5.0 mg/dL (PT threshold) 10.8% (95% CI 6.1, 18.6) had a UB Z-score >85th percentile.
Conclusion(s): For ELBW newborns a single elevated UB at age 5±1 days increased the risk of severe NDI. Even with aggressive PT a substantial proportion were exposed to potentially neurotoxic UB levels. There is an urgent need to bring UB measurement from the bench to the bedside. Prospective studies including RCTs will be necessary to evaluate interventions based on UB.
.png)
.png)
