Neonatal GI Physiology & NEC
Neonatal GI Physiology & NEC 5: Predicting Necrotizing Enterocolitis, Gut Health, and Oral Feeding
101 - Machine Learning for the Prediction of Necrotizing Enterocolitis in Preterm Infants
Publication Number: 101.237

Rosa Verhoeven, MSc (she/her/hers)
PhD Candidate
University Medical Center Groningen
Amersfoort, Utrecht, Netherlands
Presenting Author(s)
Background: Necrotizing enterocolitis (NEC) is the most devastating gastrointestinal disease seen in neonatal intensive care units (NICUs). Despite several known risk factors such as low gestational age (GA) and birth weight (BW), physicians are unable to accurately predict which child develops NEC.
Objective: This study aimed to see if machine learning (ML) could provide support by training models on static variables and vital time series to predict NEC, and to determine which vitals are most relevant for this prediction.
Design/Methods:
Logistic regression (LR), support vector machine (SVM) and extreme gradient boosting (XGBoost) models were trained on data from preterm patients that were admitted to our NICU between Jan 2018 and Dec 2022. Infants with a GA below 30 weeks who survived more than 7 days without NEC were included, resulting in a group of 267 patients of whom 32 eventually developed NEC Bell’s stage >1 (Figure 1). The control group was randomly downsampled to different NEC:control ratios (from 1:1 to 1:4) to determine the effect of class imbalance on model performance.
Input variables were static factors (GA, BW, and sex) and vital data of the first 5 days after birth (heart rate, respiratory rate, SpO2, cerebral and splanchnic oxygen saturation). Time Series FeatuRe Extraction of basis of Scalable Hypothesis tests (TSFRESH) [1] was used to automatically extract statistically significant static features from these time series. These TSFRESH features were evaluated with regard to the relative contribution of each vital to determine their importance. In addition, we present F1 scores and precision-recall (PR) AUCs of each model, portraying predictive power.
Results:
Most TSFRESH features were derived from the splanchnic oxygen saturation (M=47.79%, Table 1), and least from the respiratory rate (M=9.08%). The average prediction of the models on all features declined with an increased class imbalance, but on average the best results were obtained by LR (F1=0.89 and PR-AUC=0.95 in 1:1 ratio, F1=0.67 and PR-AUC=0.71 in 1:4 ratio, Table 2).
Conclusion(s):
We were able to predict NEC using ML with an F1 predictive power up to 0.89 using LR, with splanchnic oxygen saturation as an important vital predictor. However, predictive power diminished with the inclusion of more controls, which warrants more data of patients with NEC in future endeavors. Yet, the current study already showed that ML could be used to predict NEC early after birth in preterm infants using vital data. Doing so, individualized measures can be taken to prevent NEC, and with that NEC-related morbidity and mortality.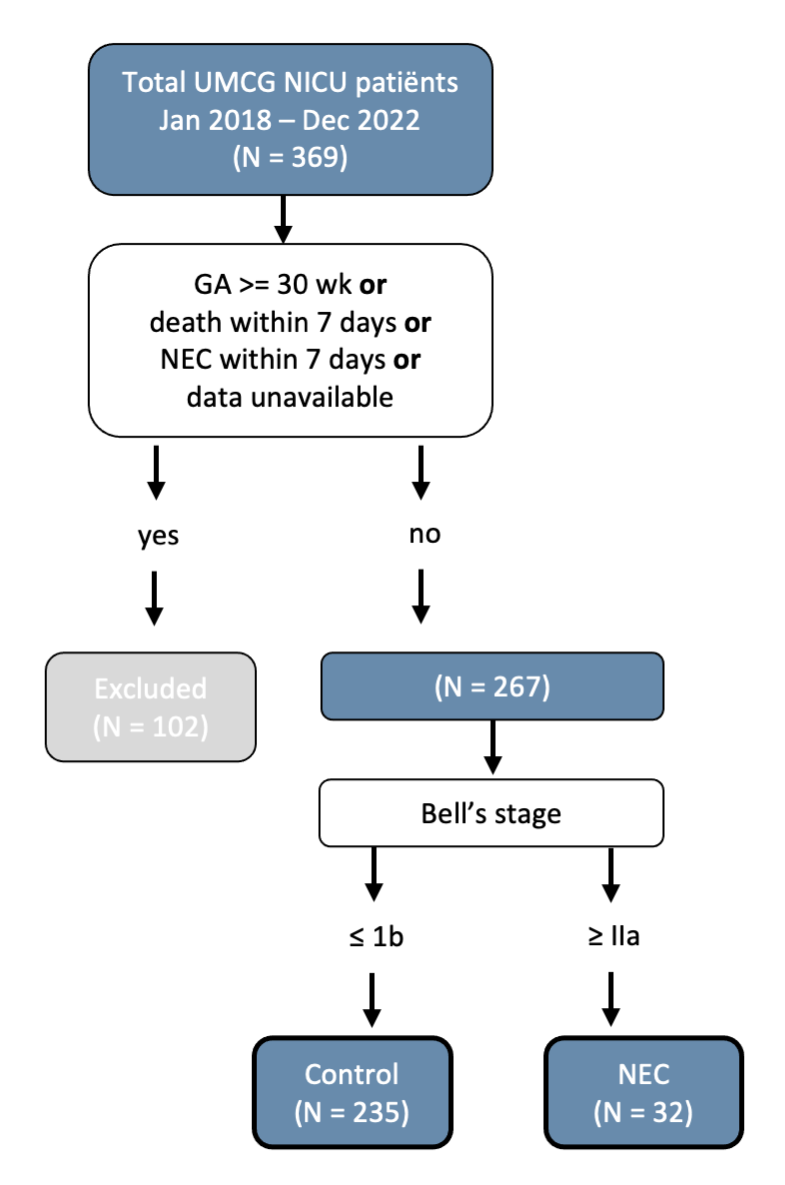
.png)
.png)
