Neonatal General
Neonatal General 4: GI-Nutrition-Growth
682 - Population-based Screening Strategies for Biliary Atresia in the Newborn: A Systematic Review
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 682
Publication Number: 682.232
Publication Number: 682.232
Srirupa Hari Gopal, Baylor College of Medicine, Houston, TX, United States; Rema A. Zebda, Baylor College of Medicine, Houston, TX, United States; Kristin Borovsky, Baylor College of Medicine, Houston, TX, United States; Arvind Mohan, University of Pennsylvania, Bellaire, TX, United States; Sanjiv Harpavat, Texas Children's Hospital, Houston, TX, United States; Mohan Pammi, Baylor College of Medicine, Bellaire, TX, United States

Srirupa Hari Gopal, MBBS
Fellow Physician
Baylor College of Medicine
Houston, Texas, United States
Presenting Author(s)
Background: Biliary atresia (BA) is the leading indication for pediatric liver transplant. The rapid course of BA can be slowed with the Kasai portoenterostomy (KPE), a procedure that establishes bile flow by creating a liver-intestine anastomosis. Timing of diagnosis and surgery impacts the overall prognosis in BA. Newborn screening for BA will facilitate early diagnosis and improve outcomes.
Objective: To perform a systematic review on the accuracy of population-based screening strategies for BA in the newborn. The primary outcome was to summarize the accuracy of various screening strategies including direct/conjugated bilirubin (DB/CB), stool color card test (SCC), serum bile acids, free carnitine and urinary sulfated bile acids (USBA) for the early diagnosis of BA.
Design/Methods: We performed the review based on PRISMA-DTA guidelines and recommendations by the Cochrane Screening and Diagnostic Methods Group. Methodological quality was assessed using Quality Assessment of Diagnostic-Accuracy Studies (QUADAS-2). Forest plots were created for sensitivity and specificity comparisons. Certainty of evidence was rated using the GRADE scale.
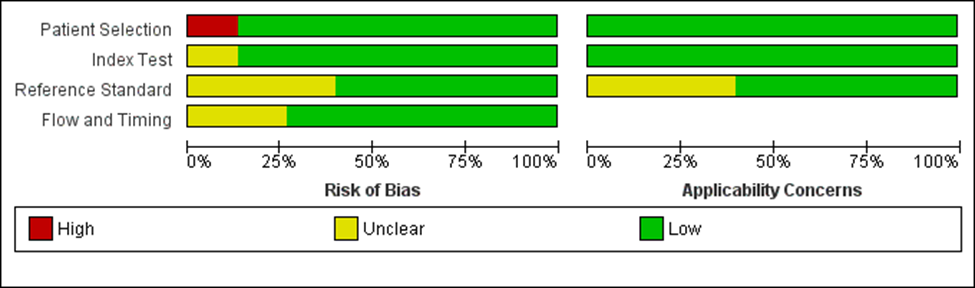
Results: We included 14 studies, which reported 5 population-based screening strategies but screening using USBA, serum bile acids and free carnitine were reported in only 1 study. DB/CB had a sensitivity of 1.00 for detecting BA in all 4 included studies and specificity ranged from 0.99 to 1.00. DB/CB had the advantage of screening in the first few days of life and being incorporated along with other newborn screening strategies. SCC screening had sensitivity ranging from 0.5 to 1 and specificity of 1.00 in the 7 included studies. SCC as a population based screening strategy was usually screened at home and followed up in the outpatient clinic at about a month of age (Figure 1). QUADAS-2 assessment noted that most included studies were methodologically adequate except for high risk of bias for patient selection in one study and uncertain risks for reference standard in 2 studies (Figure 2). GRADE scale showed a moderate certainty of evidence for both DB/CB and SCC as a screening tool for BA in newborn.
Conclusion(s): Population based newborn screening strategy using DB/CB screening was 100% sensitive with high specificity and can be performed in the first few days after birth. SCC screening had a very high specificity but performed much later at a month of age. Further research is warranted to investigate the cost-effectiveness of various screening strategies, alone or in combination, for BA and the effects of early diagnosis on, timing of KPE and the need for liver transplant.
.png)
