Neonatal Hematology & Bilirubin Metabolism
Neonatal Hematology & Bilirubin Metabolism 1: Bilirubin
113 - Diagnostic accuracy of portable, handheld, point-of-care tests versus laboratory-based bilirubin quantification in neonates: A systematic review and meta-analysis
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 113
Publication Number: 113.238
Publication Number: 113.238
Lauren EH. Westenberg, Erasmus MC, Rotterdam, Zuid-Holland, Netherlands; Andrei Tintu, Erasmus MC, Rotterdam, Zuid-Holland, Netherlands; Wichor M.. Bramer, Medical Library, Rotterdam, Zuid-Holland, Netherlands; Peter H.. Dijk, University Medical Center Groningen, Groningen, Groningen, Netherlands; Eric Steegers, Erasmus MC, Rotterdam, Zuid-Holland, Netherlands; Irwin Reiss, Erasmus MC, Rotterdam, Zuid-Holland, Netherlands; Jasper V. Been, Erasmus MC Sophia Children's Hospital, Rotterdam, Zuid-Holland, Netherlands; Christian V. Hulzebos, Beatrix Children's Hospital, University Medical Center Groningen, Groningen, Groningen, Netherlands

Lauren EH Westenberg, MD (she/her/hers)
PhD-Candidate
Erasmus MC
Rotterdam, Zuid-Holland, Netherlands
Presenting Author(s)
Background: Quantification of bilirubin in blood is essential for early diagnosis and timely treatment of neonatal hyperbilirubinemia. Handheld point-of-care (POC) devices may overcome the current issues with conventional laboratory-based bilirubin (LBB) quantification. Findings from this review will inform health-care professionals about the validation of POC bilirubin devices in everyday neonatal care.
Objective: We systematically evaluated the reported diagnostic accuracy of POC devices as compared to LBB quantification.
Design/Methods: A systematic literature search was conducted in six electronic databases up to the 5th of December 2022. Studies were included if they were prospective cohort, retrospective cohort or cross-sectional studies and reported on the comparison between POC device(s) and laboratory-based measurement for quantifying bilirubin in neonates aged 0–28 days. POC devices needed to be portable, handheld, and provide a result within 30 minutes. Risk of bias was assessed using the QUADAS-2 tool. Primary outcome were the mean difference (MD) and limits of agreement (LOA) in bilirubin level between the POC device and laboratory-based quantification using a novel meta-analysis technique to pool data from Bland-Altman plots. Secondary outcomes were: 1) turnaround time, 2) blood volumes, and 3) percentage of failed quantifications.
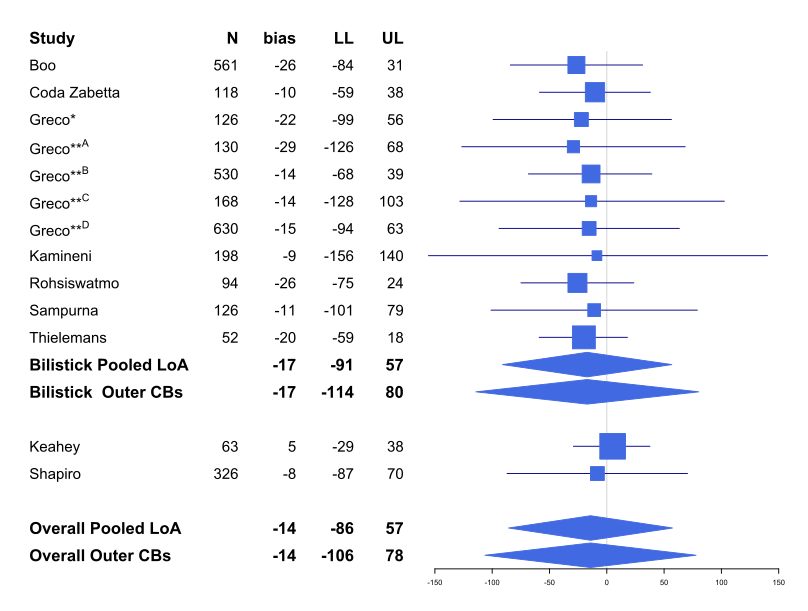
Results: Ten studies met the inclusion criteria (nine cross-sectional studies; one prospective cohort study). Three studies were considered high risk of bias. Eight studies evaluated the Bilistick; two studies the BiliSpec as the index test. A total of 3122 paired measurements showed a pooled MD of –14 µmol/L with a pooled 95% confidence interval ranging from –106 to 78 µmol/L. For the Bilistick the pooled MD [95%CI] was –17 [–114 to 80] µmol/L (Figure 1). Point-of-care devices were up to ~ 60 times faster to obtain results as compared to laboratory-based quantification, whereas blood volume needed was 40−60 times less. Bilistick was more likely to have a failed quantification as compared to laboratory-based bilirubin.
Conclusion(s): This is the first systematic review to assess the diagnostic properties of two currently available POC bilirubin devices. Although handheld point-of-care bilirubin devices allow for fast bilirubin measurements, the percentage of failed quantifications and their imprecision for measurement of neonatal bilirubin need to be improved. Future research should focus on assessing the proportional bias of the POC bilirubin devices. The clinically relevant limits of bilirubin quantification should be defined a priori in international guidelines.