Neonatal Nephrology/AKI
Neonatal Nephrology/AKI 2
238 - Cefotaxime and Pentoxifylline Mitigate Acute Kidney Injury and Staining for Kidney Injury Markers in Escherichia coli-septic Neonatal Mice
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 238
Publication Number: 238.243
Publication Number: 238.243
Esther M. Speer, Stony Brook Children's Hospital, Stony Brook, NY, United States; Atilade Adedayo. Adedeji, Stony Brook University, Stony Brook, NY, United States; Amrendra Singh, Stony Brook Children's Hospital, Port Jefferson, NY, United States

Esther M. Speer, MD
Associate Professor of Pediatrics
Stony Brook Children's Hospital
Stony Brook, New York, United States
Presenting Author(s)
Background: Neonatal sepsis triggers a host inflammatory response that contributes to increased mortality and acute kidney injury (AKI) in term and preterm neonates, for which no effective treatment exists. The phosphodiesterase inhibitor pentoxifylline (PTX) has demonstrated renal protection from ischemia and inflammation in adult rodents. We hypothesized that addition of PTX to antibiotics may diminish histological kidney injury in a murine neonatal sepsis model.
Objective: To determine histological and immunohistochemical changes in murine neonatal AKI, and the potential protective effects of addition of PTX to antibiotics.
Design/Methods: 7 days old C57BL/6J mice (N=37) were injected intraperitoneally (ip) with E. coli K1 at 105 colony forming units per gram weight or an equal volume of saline for controls. After 1.5 hours (h), septic pups randomly received ip saline, cefotaxime (CEF), or CEF with PTX (CEF+PTX), while controls received saline. Pups were euthanized 12 h after sepsis initiation. Formalin-fixed paraffin-embedded kidneys were stained with hematoxylin-eosin (H&E), periodic acid-Schiff (PAS), and antibodies against neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1) and lotus lectin. Findings were analyzed with 1-way ANOVA or Kruskal-Wallis tests.
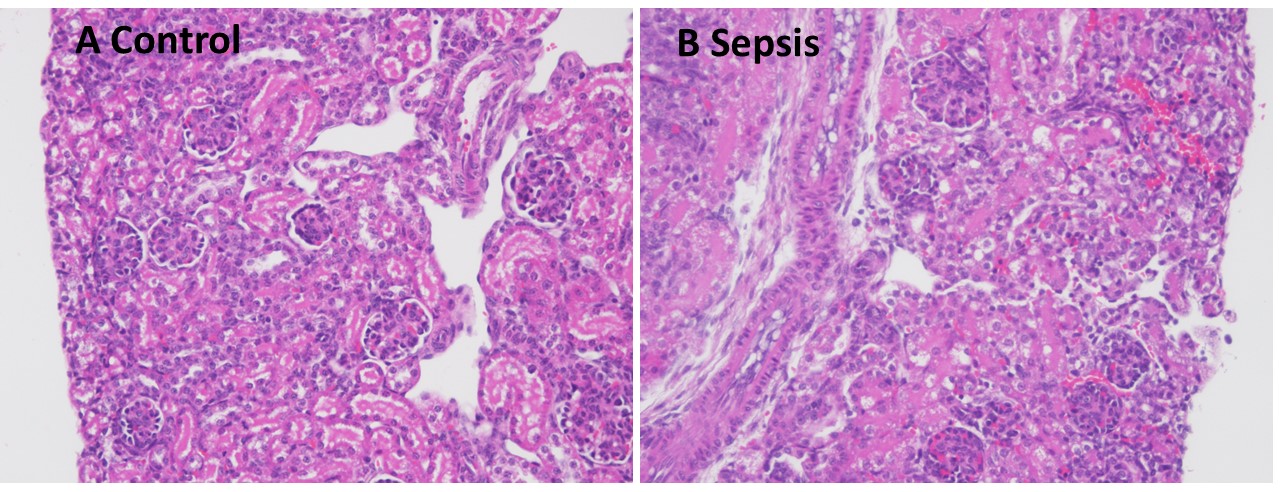
Results: 3 septic non-survivors with markedly changed postmortem images (H&E and PAS) were excluded from further analyses. Compared to controls, septic mice showed focal areas of glomerular congestion and interstitial hemorrhage (Fig. 1), which were absent in several pups treated with CEF or CEF+PTX. Renal cortical mean fluorescent NGAL-staining was significantly higher in septic vs control mice (p< 0.01). CEF further increased NGAL-staining compared to untreated septic mice (p< 0.01), whereas addition of PTX to CEF abolished the CEF-induced increases in NGAL-staining (Fig. 2). Likewise, CEF and CEF+PTX significantly diminished renal cortical mean KIM-1-staining compared to untreated septic mice, whereas staining of brush borders with lotus lectin did not show any group differences (Fig. 3). The imaging findings were consistent with our previously reported significantly higher concentrations of injury and inflammation markers in homogenized renal tissue from septic vs control neonatal mice (Speer, PAS 2021).
Conclusion(s): Newborn E. coli sepsis leads to focal renal injury and increased staining for kidney injury markers, which may be mitigated with antibiotic and PTX treatment.
.jpg)
.jpg)
