Breastfeeding/Human Milk
Breastfeeding/Human Milk 4: Breastfeeding and Milk Provision
33 - Sustained Decreased Special Care Nursery Admissions and Increased Exclusive Breast Milk Feedings for Newborns At-Risk for Hypoglycemia
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 33
Publication Number: 33.202
Publication Number: 33.202
Erwin T. Cabacungan, Medical College of Wisconsin, Wauwatosa, WI, United States; kristin Ahrens, Froedtert, West Bend, WI, United States; Regina H. Hirt, Froedtert Menomonee Falls Hospital, Menomonee Falls, WI, United States; Pamela Johnston, Froedtert Menomonee Falls Hospital, Menomonee Falls, WI, United States; Sarah Mess, Medical College of Wisconsin, Jackson, WI, United States; Leah Witte, Froedtert Health, Allenton, WI, United States; Staci Bohling, Froedtert West Bend Hospital, West Bend, WI, United States

Erwin T. Cabacungan, MD, MPH
Associate Professor
Medical College of Wisconsin
Wauwatosa, Wisconsin, United States
Presenting Author(s)
Background: We previously decreased at-risk for hypoglycemia newborns' admissions to our special care nurseries (SCN) by using glucose gel and increased their rates of exclusive breast milk feedings by maternal antenatal breast milk expression (ABE) at 36 weeks' gestation in low-risk diabetic women or supplementation with pasteurized human donor milk (PDHM). However, data showing sustained change using these interventions is lacking.
Objective: To sustain at-risk for hypoglycemia newborn SCN admissions by an average of ≤1 per 6 months and their continued exclusive breast milk feeding rates by an average of >60% per month over a 1 ½ year period.
Design/Methods: This QI project was implemented at two Community Hospital nurseries (Sites and B). Baseline and action data (2013-2020) and "sustainability" data (1/2021-6/2022) were obtained. The plan-do-study-act (PDSA) cycles included educating stakeholders on the advantages of breastfeeding and developing protocols and flow charts. Outcome measures included % of admissions to the SCN, % of exclusive breast milk feedings, and increased consecutive exclusive breast milk feeding for at-risk newborns for hypoglycemia who got glucose gel. Process measures included % of the correct duration of blood sugar screenings and % of documented expressed breast milk amount from mothers who underwent ABE. Balancing measures included untoward events (i.e., preterm labor). Statistical process charts were used.
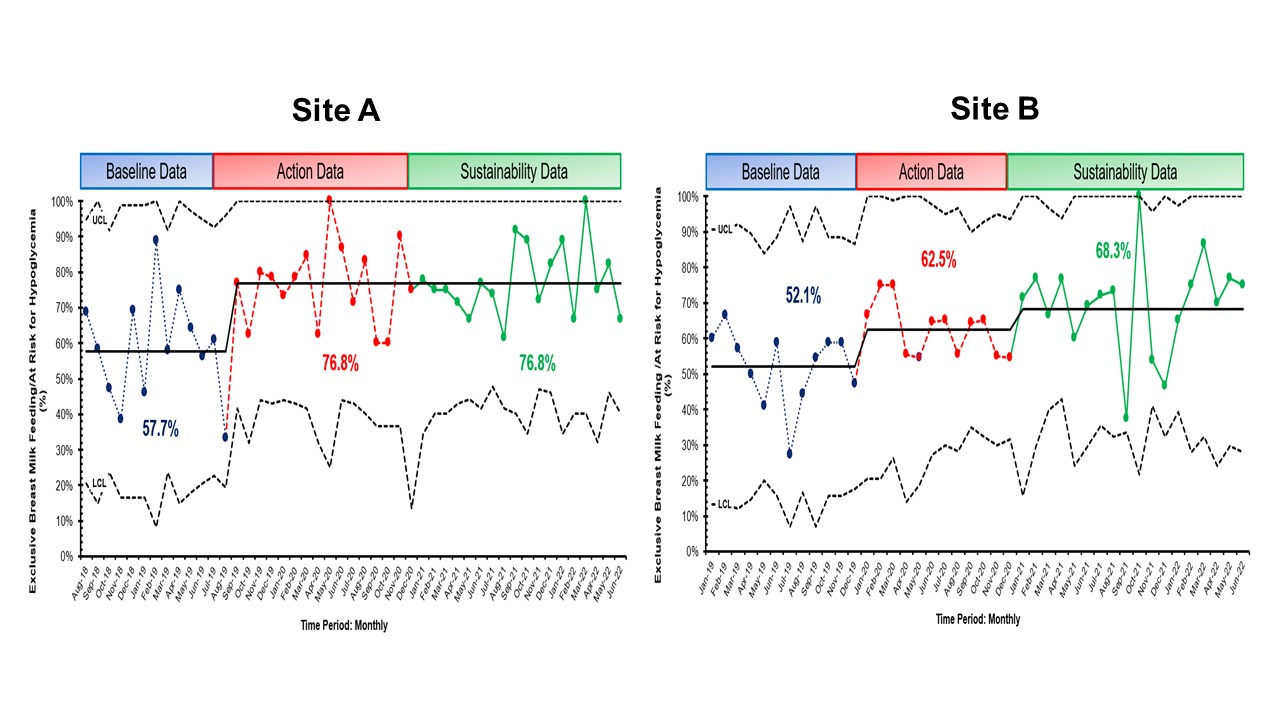
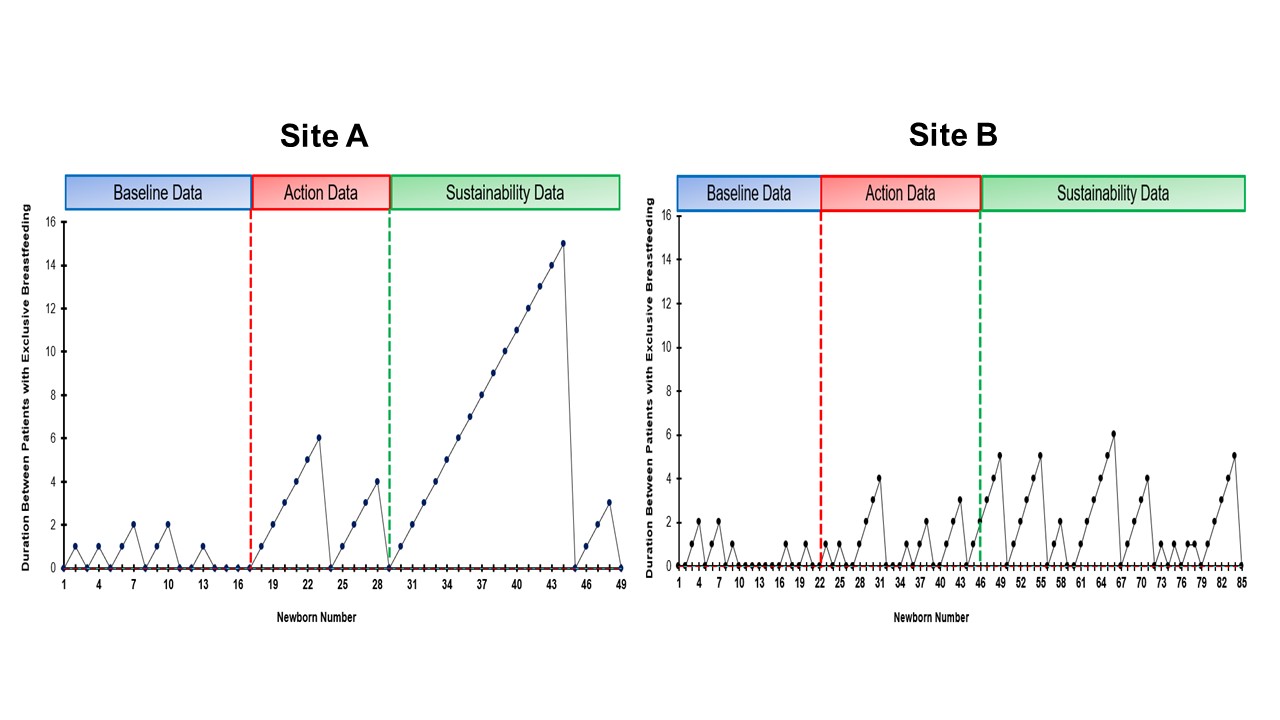
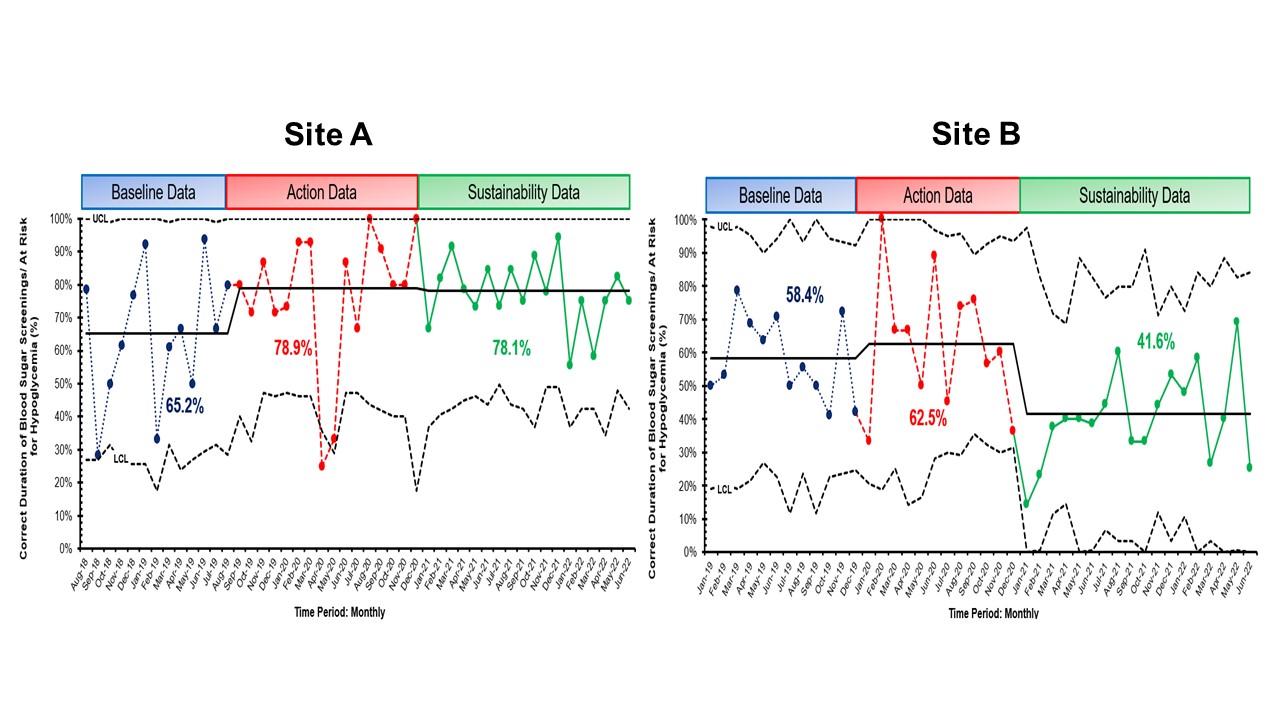
Results: At both sites, there was sustained decreased SCN admission (Site A – 0.3 admissions/6 months, Site B -0.7 admissions/6 months) and >60% exclusive breastfeeding rates (Site A – 76.8% and Site B – 68.3%, Figure 1). There were 49 at-risk for hypoglycemia newborns who got glucose gel in Site A and 85 newborns in Site B (Figure 2). Site A had a peak of 15 consecutive breast milk-feeding newborns, and Site B had 6 newborns. The % correct duration of blood sugar screenings decreased by 0.8% for Site A (78.1%) but markedly decreased by 20.9% for Site B (41.6%). There was also 97% documentation of breast milk amount from mothers who performed ABE (n = 136). Newborns of mothers who did ABE had no admissions to the SCN. For balancing measures, there were no untoward events.
Conclusion(s): We showed a sustained change of decreased SCN admissions and increased exclusive breast milk feeding rates for infants at risk for hypoglycemia using glucose gel, ABE, and supplementation with PDHM. In addition, regularly updated process control boards are needed to improve culture change among stakeholders and for the staff to see up-to-date QI progress.