Immunizations/Delivery
Immunizations/Delivery 1
602 - A Mixed Methods Approach to Understanding NYC Parental Attitudes on the COVID-19 Pediatric Vaccine
Publication Number: 602.224
- NV
Nita Vangeepuram, MD, MPH (she/her/hers)
Associate Professor
Icahn School of Medicine at Mount Sinai
New York, New York, United States
Presenting Author(s)
Background:
In late 2021, the FDA provided emergency use authorization for the COVID-19 vaccination in children ages 5+. While hesitancy was anticipated, we did not yet know what factors would strongly predict intentions to vaccinate amongst parents and caregivers.
Objective: To conduct a mixed methods assessment of parents/caregivers’ attitudes towards childhood COVID-19 vaccination in NYC.
Design/Methods:
From January to March of 2022, we surveyed 94 parents about their vaccination status, sources of trusted information, motivations to vaccinate themselves, and perceived safety/effectiveness of the vaccine against breakthrough infections and variants for themselves and children. We conducted bivariate analyses to identify factors significantly associated with pediatric vaccination intention.
In English and Spanish focus groups with 44 adults, we asked about their thoughts on the pediatric vaccine. We translated, transcribed, and uploaded transcripts to Dedoose for coding and analysis.
Results:
Table 1 provides participant demographics. Factors significantly associated with parent/caregiver’s report of child vaccination completion/intention include their own vaccination status, safety-oriented vaccine motivations, trust in doctors as a source of information, and a reported annual household income of $50,000+ (Table 2). In contrast to the quantitative data, qualitative analysis (Table 3) showed higher degrees of pediatric vaccine hesitancy regardless of personal vaccination status. Common concerns included children’s inabilities to provide consent, short and long-term side effects, the sensitivity of children’s bodies to the vaccine when compared to adults, children’s relative low risk, and the lack of anecdotal evidence within their personal networks. Despite acknowledging fear and breakthrough infections, those who supported child vaccination cited increasing potential exposures, vaccine mandates, school-based access to vaccines, and offering children the same protection as adults.
Conclusion(s): Our survey results align with other recent studies examining parental attitudes towards the pediatric COVID-19 vaccine and predictors of uptake. However, results from our focus groups indicate that there is a depth of nuance in how adults, including parents, caregivers and grandparents, weigh the risks and benefits of the vaccine for children. As more information about COVID-19 and the vaccine becomes available, mixed-methods research may provide a more complete understanding of evolving perceptions.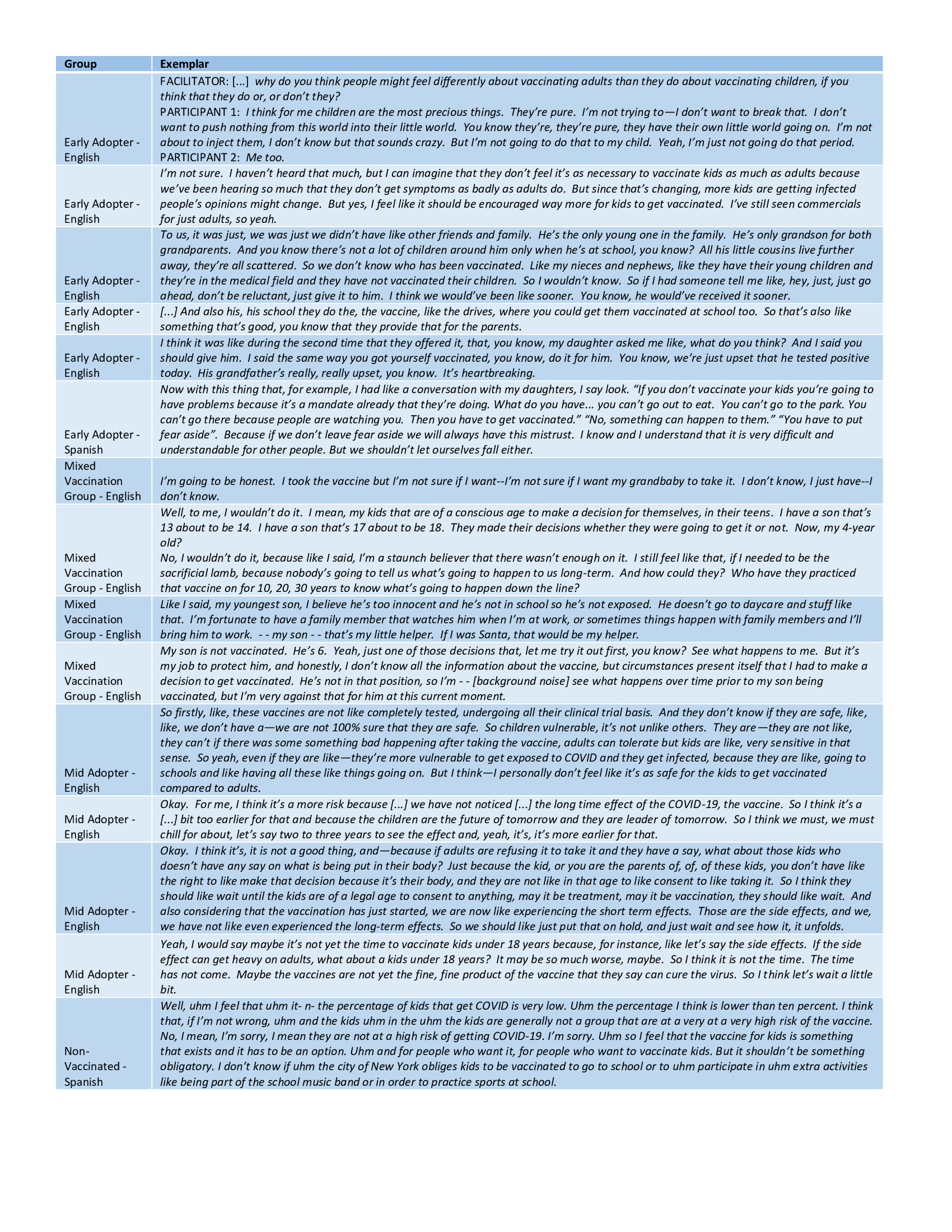
.jpg)
.jpg)
