Neonatal Clinical Trials
Neonatal Clinical Trials 1
249 - Randomized Trial of Routine vs. Selective Human Milk Fortification in Extremely Preterm Infants
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 249
Publication Number: 249.127
Publication Number: 249.127
Swati Murthy, State University of New York Upstate Medical University, Syracuse, NY, United States; Boura'a Bou Aram, SUNY UPSTATE Medical University, Syracuse, NY, United States; Steven J. Gross, State University of New York Upstate Medical University, Syracuse, NY, United States

Swati Murthy, MD (she/her/hers)
Clinical Assistance Professor of Pediatrics
State University of New York Upstate Medical University
Syracuse, New York, United States
Presenting Author(s)
Background: Fortified maternal breast milk is considered the ideal feeding for extremely preterm infants. However, optimal timing, complications, and long-term benefits of fortification are unclear.
Objective: To compare feeding-related morbidities, growth and developmental outcomes in extremely preterm infants fed preterm milk either with fortification added routinely or with fortification added subsequently only for poor weight gain.
Design/Methods: A prospective randomized controlled trial of infants ≤27 weeks gestational age (GA) admitted to the Regional Perinatal Center in Syracuse, NY was conducted from Feb 2020 to Sep 2022. Infants with early death, severe IVH and congenital anomalies were excluded. Trophic feeds (12 mL/kg/day) were started on day 7 of life, continued for 7 days, and then advanced by 15 mL/kg/day until 150 mL/kg/day. Infants were then randomized to ROUTINE GROUP (Prolacta+4 added and feeds maintained at 150 mL/kg/day) or SELECTIVE GROUP (no fortification and feeds advanced and maintained at 180 mL/kg/day). Thus, both groups received 120 kcal/kg/day. Infants in the SELECTIVE GROUP were subsequently fortified only for poor growth (weight gain < 15 g/kg/day x1 week) and then fed at 150 mL/kg/day. Subsequently, if weight gain remained < 15 g/kg/day, feedings in both groups were increased to 165 mL/kg/day. An in-house milk bank comprised of donors that delivered preterm allowed both groups to receive exclusive preterm human milk until 35 weeks postmenstrual age (PMA). Primary outcomes were rates of necrotizing enterocolitis (NEC), sepsis, death and anthropomorphic measures at 35 weeks PMA.
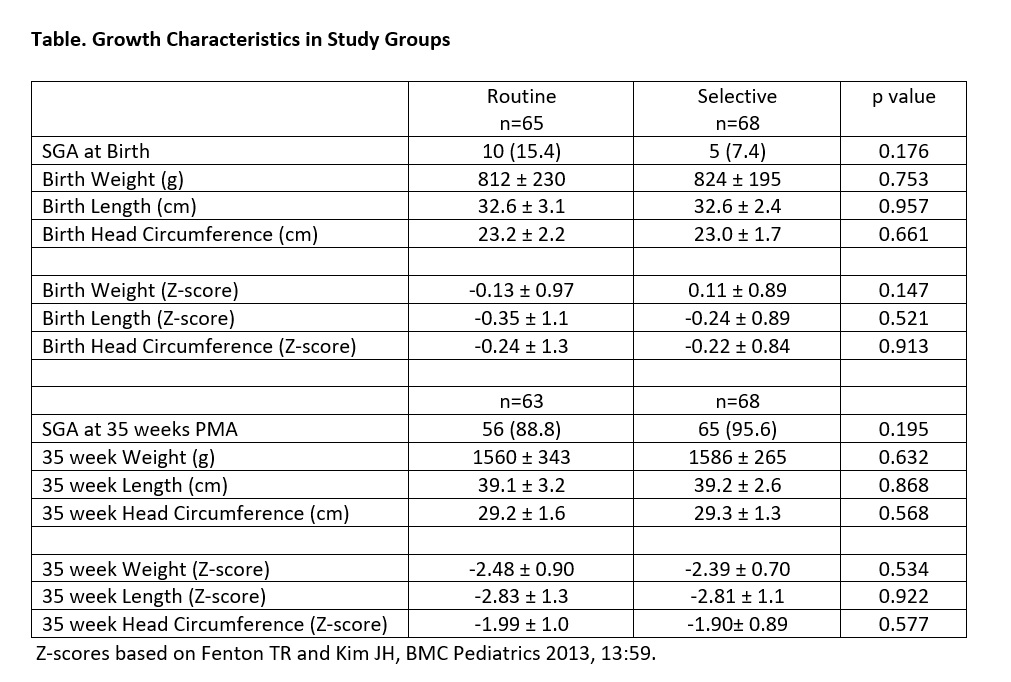
Results: Infants in the ROUTINE GROUP (n=65) and SELECTIVE GROUP (n=68) were similar for mean BW (812 ±230, 824 ±195g, respectively), GA (25.5 ±1.3, 25.3 ±1.4 weeks), inborn status (92, 85%) and 5-minute Apgar < 7 (27, 25%). While all infants in the ROUTINE GROUP received Prolacta+4, only 39 (57%) infants in the SELECTIVE GROUP received Prolacta+4 for poor weight gain; 29 (43%) infants in the SELECTIVE GROUP never had weekly weight gain < 15g/kg/day. Infants in the ROUTINE GROUP were more likely to have NEC (4/65 [6.2%] vs 0) and NEC or death (5/65 [7.7%] vs 0, P < 0.05). Sepsis after randomization occurred in 1 infant in the ROUTINE GROUP and 2 in the SELECTIVE GROUP. At 35 weeks PMA, 90% of infants were growth restricted with weight and length >2 standard deviations below the mean, regardless of fortification (Table).
Conclusion(s): Delayed human milk fortification based on weight gain reduces morbidity without significant effect on growth. Long-term follow-up for growth and neurodevelopment is on-going.