Health Equity/Social Determinants of Health
Health Equity/Social Determinants of Health 1
515 - Reporting and Representation of Participant Race and Ethnicity in NIH-Funded Pediatric Clinical Trials
Publication Number: 515.116

Lois K. Lee, MD, MPH (she/her/hers)
Associate Professor
Boston Children's Hospital, Harvard Medical School
Boston, Massachusetts, United States
Presenting Author(s)
Background:
The NIH mandates that clinical trials include diverse participant groups in a manner appropriate to reflect the populations intended for an intervention. Race and ethnicity, which are social constructs, can be used as one measure of diversity in trial participants. Understanding the representation of pediatric participants in NIH-funded clinical trials provides critical data on current practices and potential remaining gaps.
Objective:
To examine the reporting of race and ethnicity data and the distribution of participant race and ethnicity in NIH-funded pediatric clinical trials.
Design/Methods:
We conducted a cross-sectional study of pediatric (< 18 years) clinical trials funded by the NIH, conducted in the US, and with grants completed in 2017-2019. Participant race and ethnicity data were extracted from publications and ClinicalTrials.gov. For publications, we determined whether race and ethnicity were reported, how they were categorized, and the source of this information. For each racial and ethnic category, we recorded the number of participants enrolled based on data in publications and ClinicalTrials.gov. To compare the race and ethnicity of trial participants to US children, we conducted univariate logistic regression, reporting odds ratios (OR) with 95% confidence intervals (CI).
Results:
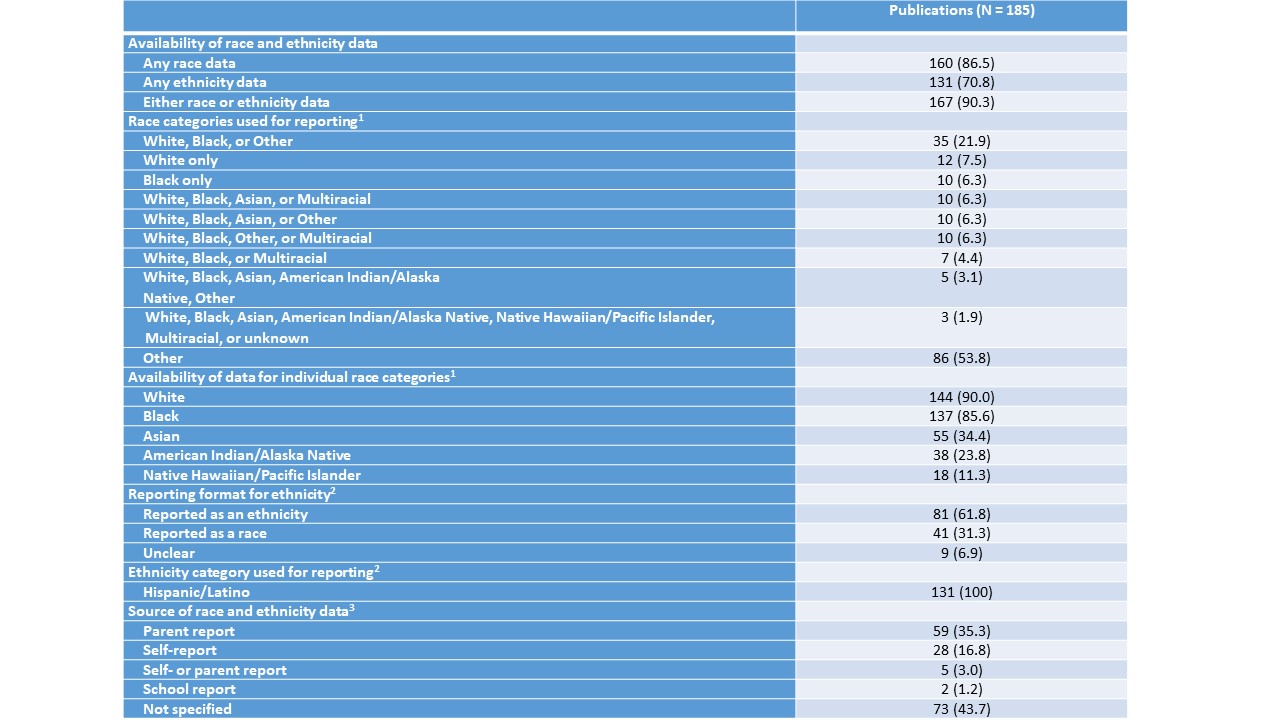
The study included 208 trials, representing 99,652 participants. Among 185 associated publications, 86% reported race data (Table 1). There was wide variation in the categories used to report race, with 43 different classifications. Data on ethnicity were provided in 71% of publications: 62% reporting as a separate ethnicity variable and 31% as a race category. The most common source of race and ethnicity reporting were parents (35%), but it was not specified in 44% of publications. The proportion of White children enrolled in clinical trials was slightly lower than in the US population (OR 0.77, 95% CI 0.76, 0.78) (Table 2). There was relative over-representation of Black (OR 1.42, 95% CI 1.40,1 .44), Asian (OR 1.79, 95% CI 1.75, 1.83), American Indian/Alaska Native (OR 2.64, 95% CI, 2.52, 2.75), and Native Hawaiian/Pacific Islander children (OR 8.07, 95% CI, 7.64, 8.41).
Conclusion(s):
NIH-funded pediatric clinical trials reported race and ethnicity using highly variable classification schemas but demonstrated good representation of marginalized populations. Funding agencies should consider prioritizing standardized collection and reporting of race and ethnicity data to support ongoing assessment of appropriate participant representation.