Neonatal Neurology: Clinical Research
Neonatal Neurology 3: Clinical 3
178 - Erythropoietin treatment during hypothermia increases blood glucose levels in infants with hypoxic-ischemic encephalopathy
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 178
Publication Number: 178.137
Publication Number: 178.137
Thomas R. Wood, University of Washington School of Medicine, Seattle, WA, United States; Ulrike Mietzsch, University of Washington School of Medicine, Seattle, WA, United States; Tai-Wei Wu, Division of Neonatology, Fetal and Neonatal Institute, Children’s Hospital Los Angeles, Department of Pediatrics, Keck School of Medicine of USC, Los Angeles, California, United States, Los Angeles, CA, United States; Bryan Comstock, University of Washington School of Medicine, Seattle, WA, United States; Dennis E.. Mayock, UWMC, Lake Forest Park, WA, United States; Patrick Heagerty, University of Washington, seattle, WA, United States; Yvonne Wu, UCSF, San Francisco, CA, United States; Sandra Juul, Sandra Juul, Bellevue, WA, United States

Tommy R. Wood, MD, PhD (he/him/his)
Research Assistant Professor
University of Washington School of Medicine
Seattle, Washington, United States
Presenting Author(s)
Background: Blood glucose (BG) dysregulation is associated with worse long-term outcome in infants with hypoxic-ischemic encephalopathy (HIE) treated with therapeutic hypothermia (TH). Erythropoietin (Epo) therapy for chronic renal failure decreases BG in adults with type 2 diabetes. The effect of Epo on BG during TH in infants with HIE is unknown.
Objective: To determine whether Epo alters BG levels in infants with moderate-severe HIE during TH.
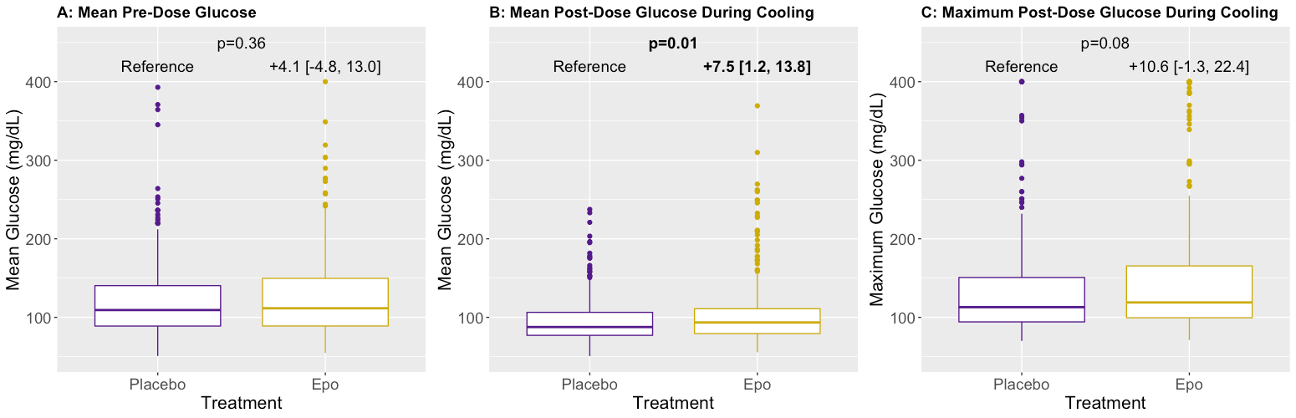
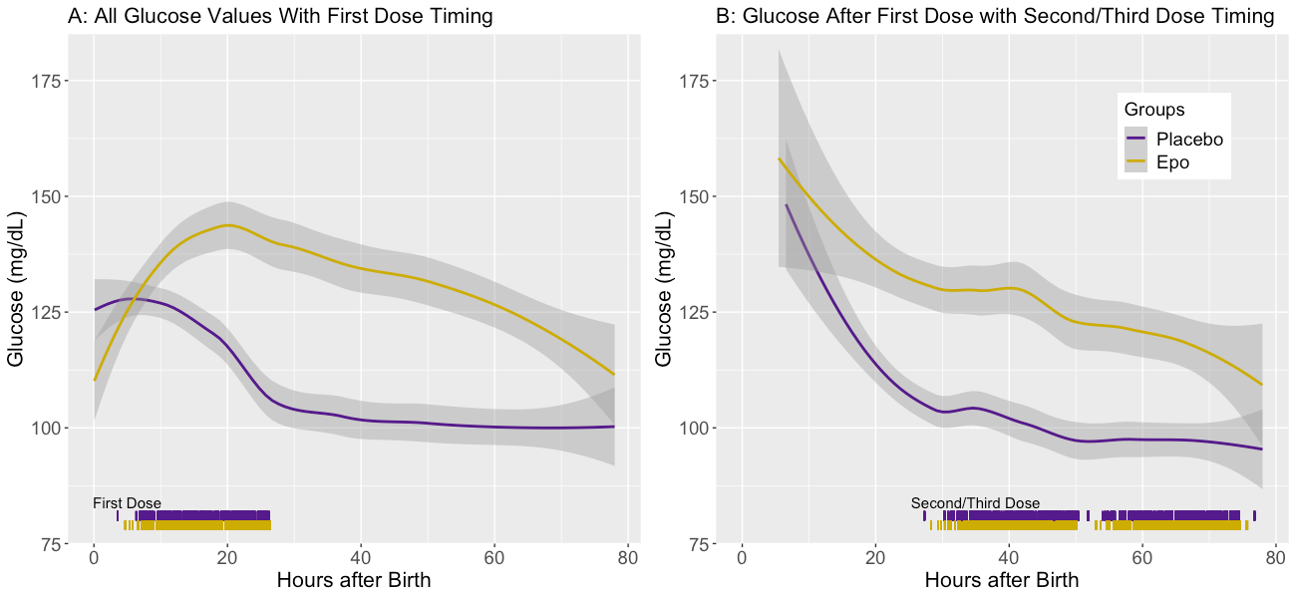
Design/Methods: We analyzed BG measurements from infants with moderate to severe HIE treated with TH and enrolled in the “High-Dose Erythropoietin for Asphyxia and Encephalopathy” (HEAL) Trial. Infants were randomized to receive either 5 doses of Epo or placebo, with the first dose given after initiation of TH but within 24h of birth. Second and third doses were given 24h and 48h after the first. 5,782 BG levels were collected from 491 infants between first study drug dose and end of TH. Mean BG throughout TH was visualized using the Locally Weighted Scatter-plot Smoother (LOESS) method. Mean BG before and after first study drug dose, and maximum BG after first study drug dose, were compared by treatment group using linear regression with robust standard errors. Adjusted odds ratio (aOR) for hyperglycemia (BG >200 mg/dL) in the Epo group was determined using logistic regression. All models were adjusted for study site and 15 potential confounders (Fig 1).
Results: Mean BG prior to first study drug dose was similar in both groups (Fig 1A). During TH, the mean and maximum BG were 97 (32) mg/dL and 138 (70) mg/dL, respectively. Mean and maximum BG during TH were around 8% higher in the Epo group; adjusted mean BG [95% CI] was 7.5 [1.2-13.8] mg/dL higher (Fig 2B, p=0.01), with a trend towards higher adjusted maximum BG (+10.6 mg/dL; -1.3-22.4, p=0.08, Fig 2C), compared to placebo. Adjusted odds of hyperglycemia was not increased in the Epo group (aOR 1.04, 95% CI 0.99-1.11, p=0.16). In the placebo group, BG generally decreased over the first 24h, whereas it rose and then declined more slowly in the Epo group (Fig 2A). Mean BG remained higher in the Epo group until the end of TH, when they were similar again (Fig 2B).
Conclusion(s): Epo treatment during TH is associated with increases in mean and maximum BG without an increased odds of hyperglycemia. Mechanisms underlying a potential interaction between Epo and TH on BG in infants with HIE require further investigation.