Neonatal Clinical Trials
Neonatal Clinical Trials 1
237 - Postnatal opioid treatment in the Eating, Sleeping, and Consoling for Neonatal Opioid Withdrawal (ESC-NOW) randomized controlled trial
Publication Number: 237.127

Lori A. Devlin, DO, MHA, MS (she/her/hers)
Professor Pediatrics
University of Louisville School of Medicine
Louisville, Kentucky, United States
Presenting Author(s)
Background:
The Eat, Sleep and Console care approach (ESC) substantially reduces the proportion of infants receiving pharmacologic treatment for neonatal opioid withdrawal syndrome (NOWS) compared to usual care (UC) with the Finnegan Neonatal Abstinence Scoring Tool. When infants with NOWS have withdrawal that is not adequately treated with non-pharmacologic interventions alone, pharmacologic treatment continues to be an important component of care. Further understanding of the influence of ESC on pharmacologically treated infants is needed to address current knowledge gaps in the field.
Objective:
Evaluate initiation and duration of opioid treatment and length of stay among pharmacologically treated infants assessed and managed with ESC verses UC.
Design/Methods:
We studied a cohort of pharmacologically treated infants within ESC-NOW, a stepped-wedge cluster randomized controlled trial. Infants were ≥ 36 weeks’ gestation and treated for NOWS at one of 26 study hospitals. Participating hospitals maintained their pre-trial practices for pharmacologic treatment including opioid type, dosing approach, and use of adjuvant medications. For each outcome, namely length of opioid treatment, time from birth until initiation of the first opioid dose, peak opioid dose, and length of hospital stay (LOS), we used generalized linear mixed-models with appropriate distribution and link function to adjust for the stepped-wedge design and maternal and infant characteristics.
Results:
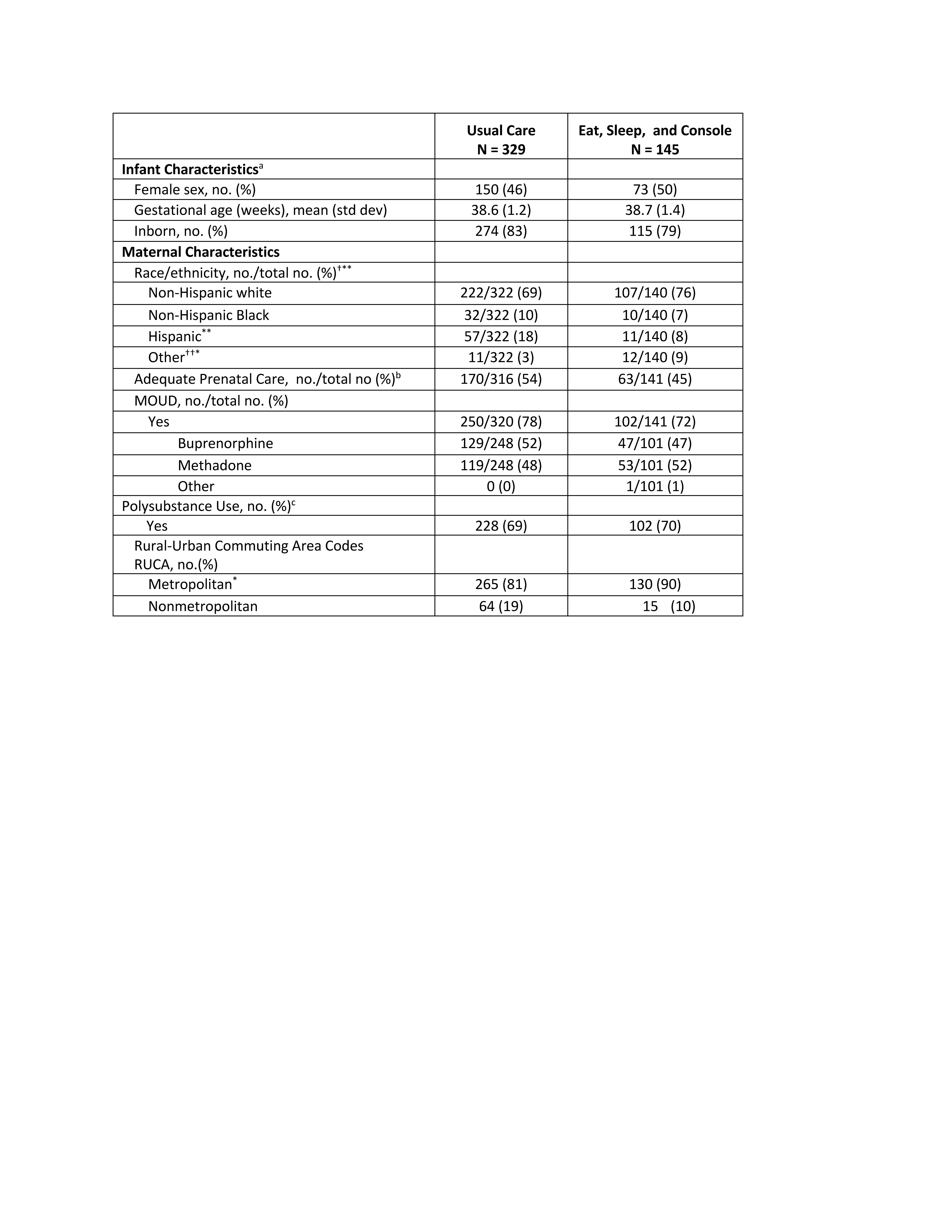
The study enrolled 1306 infants of whom 474 infants were pharmacologically treated [329/703 (47%) in the UC group and 145/603 (24%) in the ESC group]. Among these 474, demographic characteristics for the infants and their mothers were similar between the UC and ESC groups except for ethnicity and rurality (Table 1). The adjusted mean length of opioid treatment was 5.4 (95%CI:2.4-8.4) days shorter in the ESC than in the UC group (11.2 vs. 16.5 days; P=0.001). The adjusted time from birth to initiation of pharmacologic treatment was 23.1 (95%CI:8.7-37.5) hours longer in the ESC group (75.9 vs. 52.8 hours; P< 0.001) but there was no difference in mean peak opioid doses between groups (0.13 vs. 0.11 morphine mg equivalents/kg). The mean LOS was 6.2 (95%CI:3.1-9.4) days shorter in the ESC than in the UC group (16.5 vs. 22.7 days; P< 0.001).
Conclusion(s):
The ESC care approach was associated with a shorter length of opioid treatment and hospital stay among infants pharmacologically treated for NOWS. Compared to UC, use of ESC did not result in a higher peak opioid dose, though pharmacologic treatment was initiated later.