Neonatal General
Neonatal General 2
267 - Retrospective Analysis of the Clinical Outcomes Associated with Hydrocortisone versus Indomethacin Prophylaxis in Extremely Preterm Neonates
Publication Number: 267.131
.jpg)
Krishanta Maharaj, BSc, MBBS, MRCPCH, DM Pediatrics (she/her/hers)
Neonatal-Perinatal Medicine Fellow
University of Toronto
Toronto, Ontario, Canada- SG
Sandra Gerges, PharmD, MSc
Sunnybrook Health Sciences Centre
Toronto, Ontario, Canada
Presenting Author(s)
Co-Author(s)
Background:
There is limited evidence to support the choice of prophylactic indomethacin or prophylactic hydrocortisone in extremely low gestational age neonates (ELGANs).
Objective: This study aims to compare clinical outcomes following indomethacin or hydrocortisone prophylaxis to provide much needed information which may support this clinical decision.
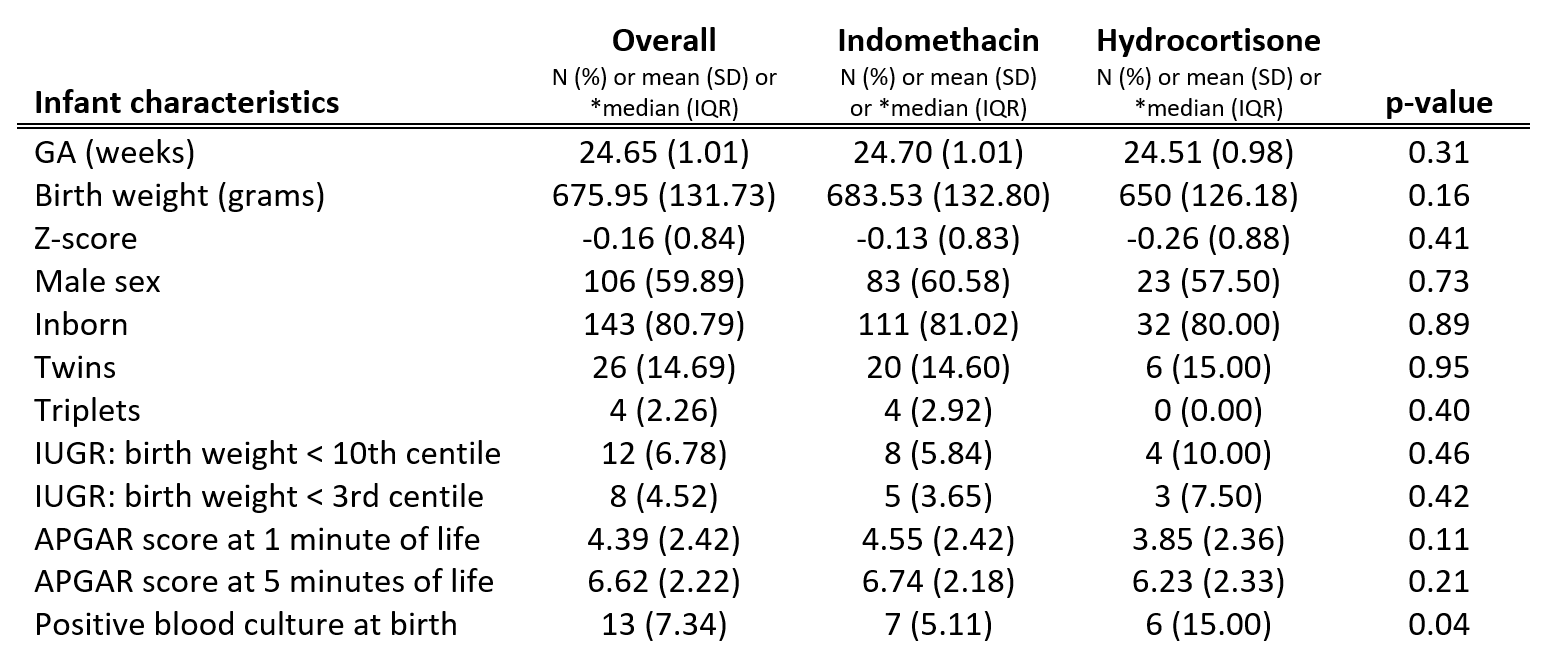
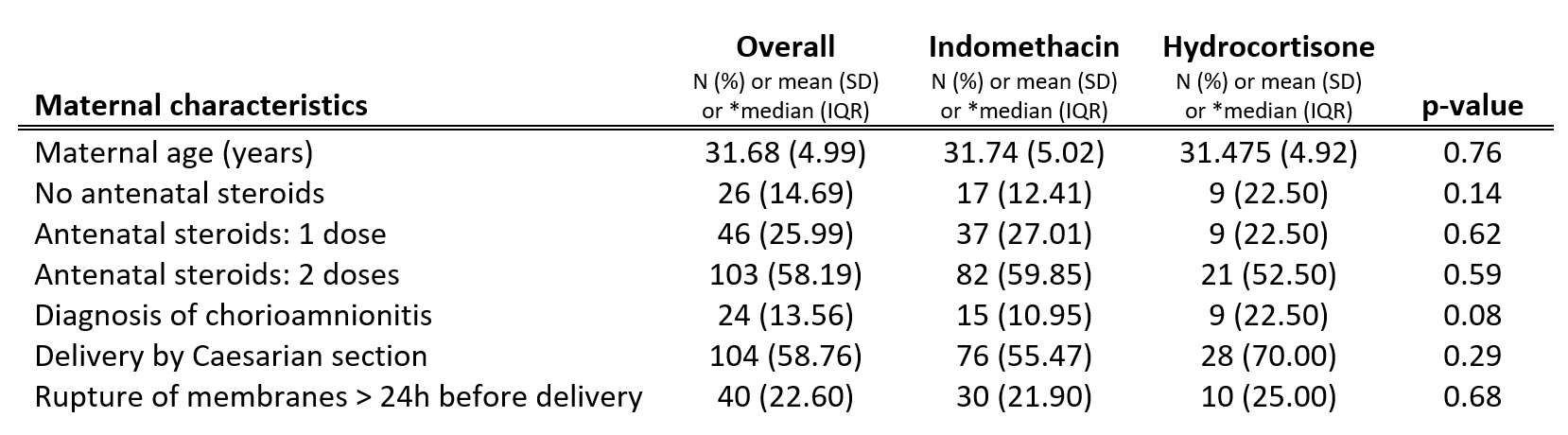
Design/Methods: A retrospective analysis was conducted in neonates less than 28 weeks gestational age (GA) and birth weight less than 1000 grams admitted to the NICU at Sunnybrook Health Sciences Centre. 137 neonates prescribed prophylactic indomethacin and 40 neonates prescribed prophylactic hydrocortisone were matched by GA and antenatal steroid exposure.
Results:
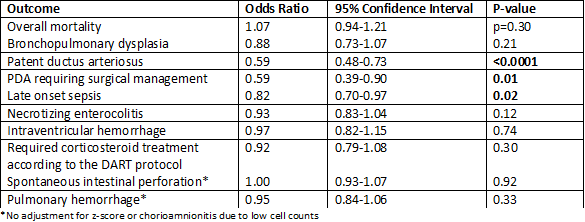
175 ELGANs were included in the outcome analysis (mean GA 24.7 ± 1.0 weeks; mean birth weight 675.9 ± 131.7 grams). Neonates treated with indomethacin had a lower incidence of PDA (OR 0.59; 95% CI 0.48-0.73; p< 0.0001), and surgical closure (OR 0.59; 95% CI 0.39-0.90, p=0.01), and lower incidence of late-onset sepsis (OR 0.82, 95% CI 0.70-0.97, p=0.02) compared with hydrocortisone treated neonates. There was no significant difference in the incidence of BPD with indomethacin versus hydrocortisone (OR 0.88; 95% CI 0.73-1.07; p=0.21).
Conclusion(s):
Among ELGANs, indomethacin was associated with significantly less PDA, PDA requiring surgical closure, and late onset sepsis than hydrocortisone. Based on the results of this study, indomethacin may be preferred over hydrocortisone to prevent common complications of prematurity. More research is needed to determine which prophylactic therapy is appropriate for specific patient populations.