Neonatal GI Physiology & NEC
Neonatal GI Physiology & NEC 1: GI Health and NEC Complications
339 - Maternal Antibiotic Treatment Dysregulates Goblet Cell Associated Antigen Passages in Neonatal Mice
Publication Number: 339.133

Shreya Gaddipati (she/her/hers)
Research Assistant
Washington University in St. Louis School of Medicine
University City, Missouri, United States
Presenting Author(s)
Background:
Intestinal goblet cell associated antigen passages (GAPs) are portals for exposure of the immune system to luminal antigens for the development of tolerogenic responses. In the neonatal period, GAPs are inhibited by epidermal growth factor receptor (EGFR) signaling. While breast milk derived EGF is the primary inhibitor of GAPs in the neonatal period, recent studies have demonstrated that the microbiota modulates active EGFR ligand availability in the intestine. Additionally, maternal antibiotic treatment (MAT) disrupts the development of the neonatal microbiome and intestinal immune development and increases the risk for sepsis in mouse models, but its effect on GAP regulation is unknown.
Objective:
We hypothesise that MAT disrupts GAP regulation in EGFR-dependent mechanism.
Design/Methods:
Neonatal mice were exposed to ampicillin and neomycin (1g/L each) in the maternal drinking water from pup day of life (DOL) 1-8. Controls were maintained on standard drinking water. To identify GAPs, rhodamine-dextran was injected into the intestinal lumen ex vivo and incubated for 20 minutes on ice. Samples were then fixed in 10% formalin buffered saline and processed for sectioning. Concentrations of EGFR ligands epidermal growth factor (EGF) and heparin-bound EGF (HB-EGF) were measured using enzyme-linked immunosorbent assay (ELISA).
Results:
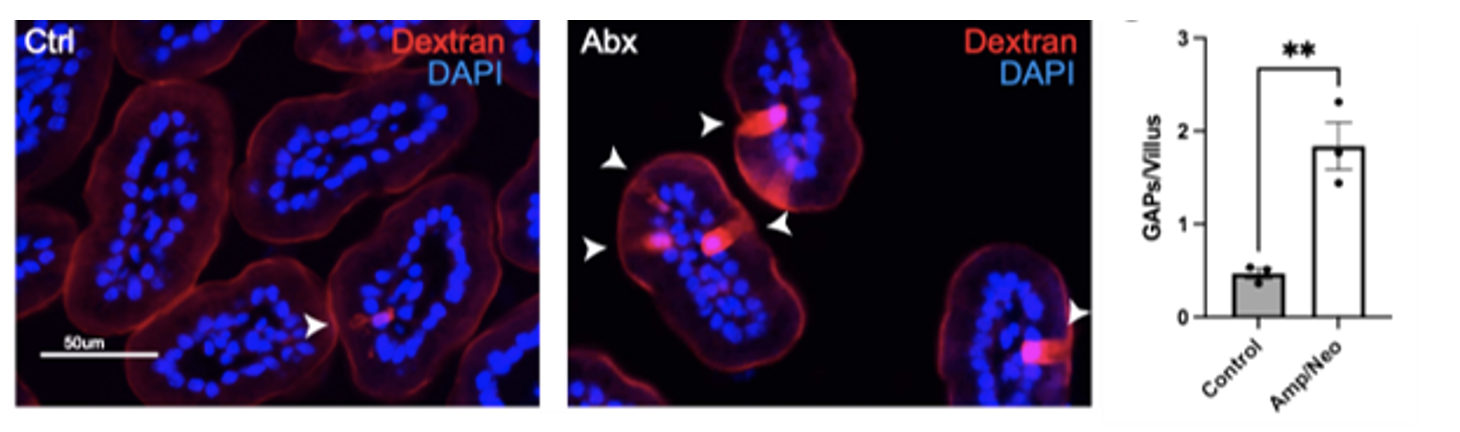
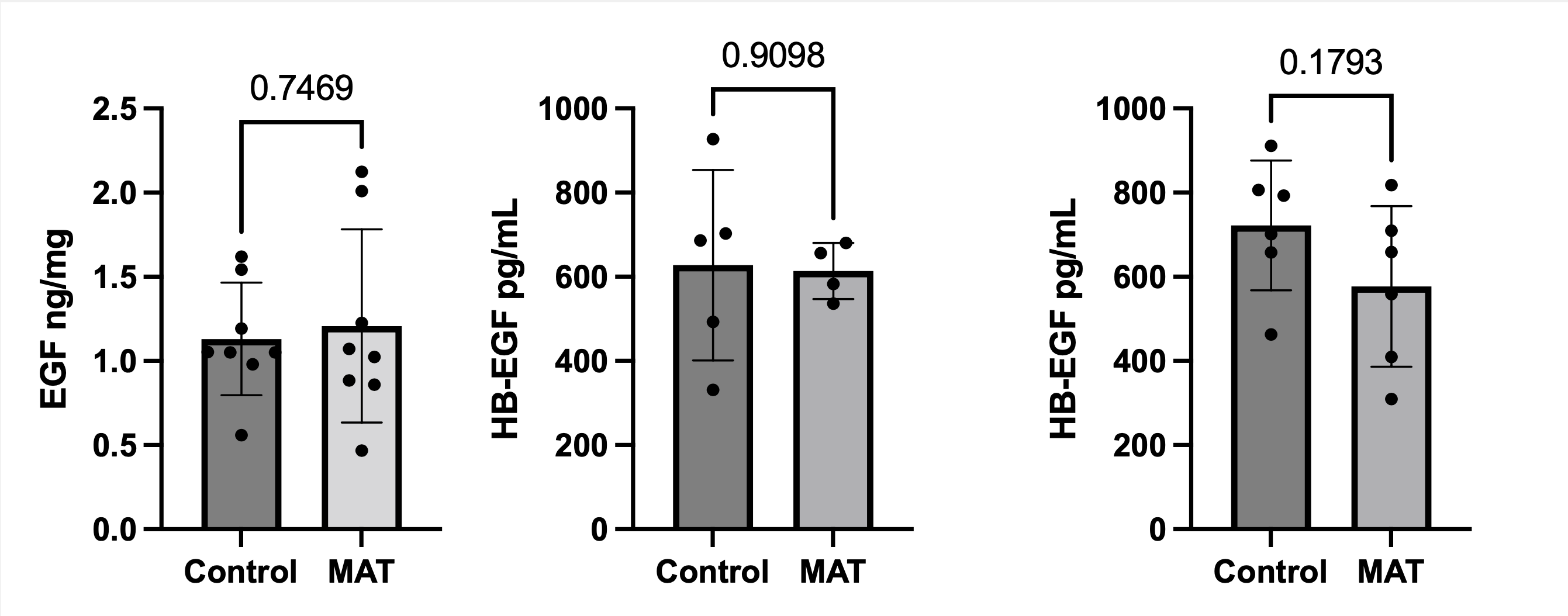
On DOL 8, MAT pups have significantly more open GAPs in the small intestine compared to controls (Figure 1). Gastric EGF and HB-EGF levels were measured to estimate growth factor levels in the breast milk. There was no difference in the EGF and HB-EGF levels in the stomach between control and MAT pups (Figure 2). Intriguingly, HB-EGF levels in the distal small intestine were lower in the antibiotic-exposed cohort compared to controls, although the difference did not reach statistical significance in our preliminary data (Figure 2).
Conclusion(s): GAPs are inappropriately opened in the small intestine of neonatal mice exposed to antibiotics. Our data suggests that this is secondary to reduced HB-EGF levels in the distal small intestine. Further studies will be necessary to determine how MAT-induced GAP dysregulation impacts neonatal sepsis risk and identify new strategies to mitigate this risk.