Neonatal Clinical Trials
Neonatal Clinical Trials 1
246 - Growth and feeding of infants with neonatal opioid withdrawal syndrome (NOWS) in the Eating, Sleeping, and Consoling (ESC-NOW) trial
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 246
Publication Number: 246.127
Publication Number: 246.127
Stephanie L. Merhar, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Lori A. Devlin, University of Louisville School of Medicine, Louisville, KY, United States; Zhuopei Hu, University of Arkansas for Medical Sciences, Little Rock, AR, United States; Songthip Ounpraseuth, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Abhik Das, RTI International, Rockville, MD, United States; Alan E. Simon, National Center for Health Statistics, SILVER SPRING, MD, United States; Brian Martin. Smith, Duke, Durham, NC, United States; Michele C. Walsh, NICHD, Bethesda, MD, United States; Jeannette Y. Lee, University of Arkansas for Medical Sciences, Little Rock, AR, United States; Rosemary Higgins, Florida Gulf Coast University, Fort Myers, FL, United States; Ward Rice, St. Elizabeth Healthcare, Edgewood, KY, United States; David A. Paul, Pediatrics, Newark, DE, United States; Jessie Maxwell, University of New Mexico, albuquerque, NM, United States; Sucheta Telang, University of Louisville, Louisville, KY, United States; Camille Fung, University of Utah, Salt Lake City, UT, United States; Tanner Wright, USF Health Morsani College of Medicine, Tampa, FL, United States; Anne Marie Reynolds, University at Buffalo, Buffalo, NY, United States; Devon Hahn, University of Oklahoma College of Medicine, Oklahoma City, OK, United States; Julie Ross, Medical University of South Carolina, Charleston, SC, United States; Jennifer McAllister, Cincinnati Children's Hospital, Cincinnati, OH, United States; Moira Crowley, Case Western Reserve University School of Medicine, Cleveland, OH, United States; Sophie Shaikh, Duke, Durham, NC, United States; Karen M. Puopolo, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, United States; Lori Christ, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Jaime Brown, MUSC and VCOM, Inman, SC, United States; Akshatha Akshatha, Kapi'olani Medical Center for Women and Children, honolulu, HI, United States; Lauren Tucker, University of Mississippi School of Medicine, Jackson, MS, United States; Karen R. McAlmon, Winchester Hospital, Winchester, MA, United States; Krishna Dummula, University of Kansas, Kansas City, KS, United States; Julie Weiner, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Jessica White, Sanford Children's Hospital, Sioux Falls, SD, United States; Meghan Howell, Tulane University School of Medicine, New Orleans, LA, United States; Sarah Newman, Nebraska Medicine, Omaha, NE, United States; Jessica Snowden, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Leslie Young, Robert Larner, M.D., College of Medicine at the University of Vermont, Burlington, VT, United States; NICHD Neonatal Research Network, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, United States; IDeA States Network, National Institutes of Health, North Bethesda, MD, United States

Stephanie L. Merhar, MD, MS (she/her/hers)
Associate Professor of Pediatrics
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background: Infants with NOWS managed with the Eat, Sleep, Console care approach (ESC) use less pharmacologic treatment and have shorter hospital stays compared to usual care (UC) with the Finnegan Neonatal Abstinence Scoring Tool. Little is known about the effects of the two approaches on feeding and weight loss during the newborn hospitalization.
Objective: Evaluate weight loss and feeding practices in infants cared for using ESC versus UC in the ESC-NOW study.
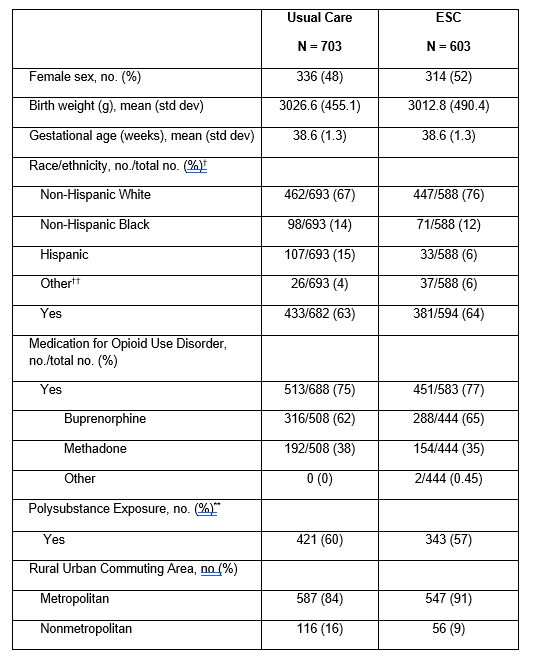
Design/Methods: ESC-NOW is a multicenter, stepped-wedge, cluster randomized trial of ESC versus UC. Infants were ≥36 weeks gestation and managed for NOWS at one of 26 study hospitals. Feeding was per site typical practice and not specified by the ESC intervention. All analyses were intention-to-treat and adjusted for stepped-wedge study design and for maternal and infant characteristics in Table 1. We used z-scores for growth parameters to account for differences in corrected gestational age at measurement. Day of life (DOL) 3 was chosen as a timepoint to assess weight loss as all infants were still in the hospital on DOL 3.
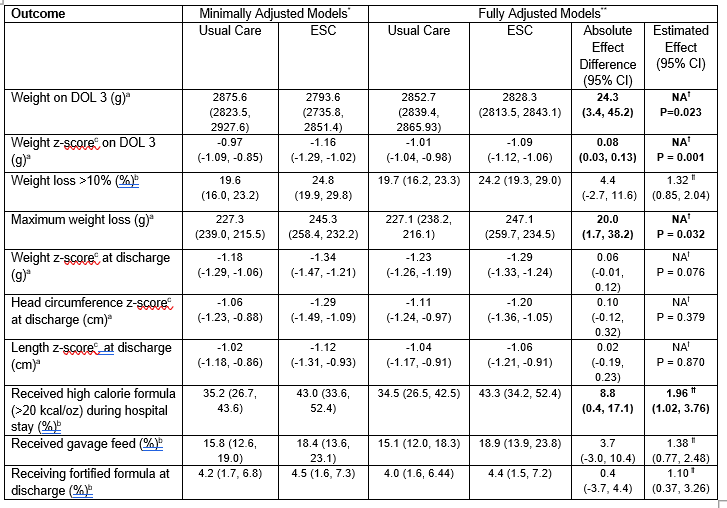
Results: This analysis included 1306 infants, 603 managed with ESC and 703 managed with UC. Infants managed with ESC had a lower weight on DOL 3 (2828.3 g vs 2852.7 g, p = 0.023), lower weight z-score on DOL 3 (-1.09 vs -1.01, p=0.001), and higher maximum weight loss during the birth hospitalization (247 g vs 227 g, p = 0.032) (Table 2). Z-scores at discharge for weight, length, and head circumference did not differ between groups. Infants in the ESC group were more likely to receive fortified formula ( >20 kcal/oz) during the hospital stay but were not more likely to receive fortified formula at hospital discharge. Outcomes did not differ between pharmacologically treated and untreated infants. There was no substantial difference in the use of gavage feeds between groups.
Conclusion(s): In this randomized controlled trial, infants managed with ESC lost more weight while in the hospital than infants managed using UC, but growth parameters at discharge were not different, which may be due to more fortified formula use during the hospital stay in the ESC group.