Neonatal Neurology: Clinical Research
Neonatal Neurology 1: Clinical 1
155 - The Impact of Prenatal Opioid Exposure on Regional Brain Volumes within the Dopaminergic Pathways
Publication Number: 155.135

Marie Sasaki, MD (she/her/hers)
Research Fellow
Tufts Medical Center
Brookline, Massachusetts, United States
Presenting Author(s)
Background:
Opioid-exposed neonates exhibit excessive yet uncoordinated feeding with unknown mechanisms. Our pilot salivary data showed greater expression of a key reward gene (dopamine receptor type 2/DRD2) that significantly correlated with higher feeding amounts in opioid-exposed neonates, evidence of the opioid effect on feeding behavior through the reward signaling. Prior brain imaging studies showed altered overall and regional brain volumes in opioid-exposed neonates. Yet, the impact of opioids on the volume of the limbic regions involved in the reward/dopaminergic circuity is not yet studied.
Objective: This study aims to examine the impact of prenatal opioids on key limbic structures involved in the dopaminergic pathways.
Design/Methods:
We prospectively recruited 26 neonates born at ≥ 34 weeks of gestational age with (n=13) and without (n=13) prenatal opioid exposure. We excluded neonates with known congenital or chromosomal anomalies, significant medical complications such as neonatal encephalopathy or cardiac disease. Non-sedated brain MRI was performed within 48 hours after birth. Raw images were reconstructed with an in-house pipeline. Using Freeview, two raters performed manual post-acquisition segmentation of key limbic structures: caudate, hippocampus, and amygdala. We did not calculate p-value due to small sample size, instead, we performed descriptive statistical analysis to compare the mean structural volumes between exposed and non-exposed neonates. Effect size was calculated to measure the magnitude of the difference between the groups.
Results:
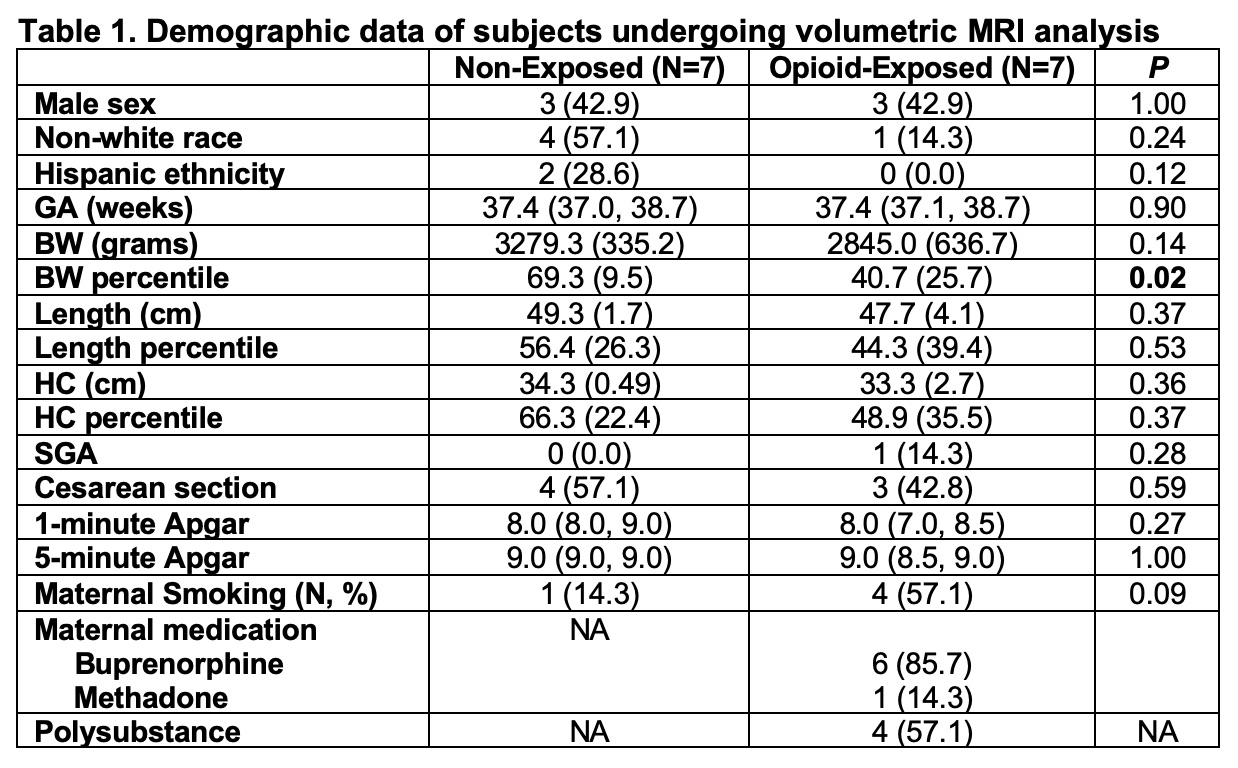
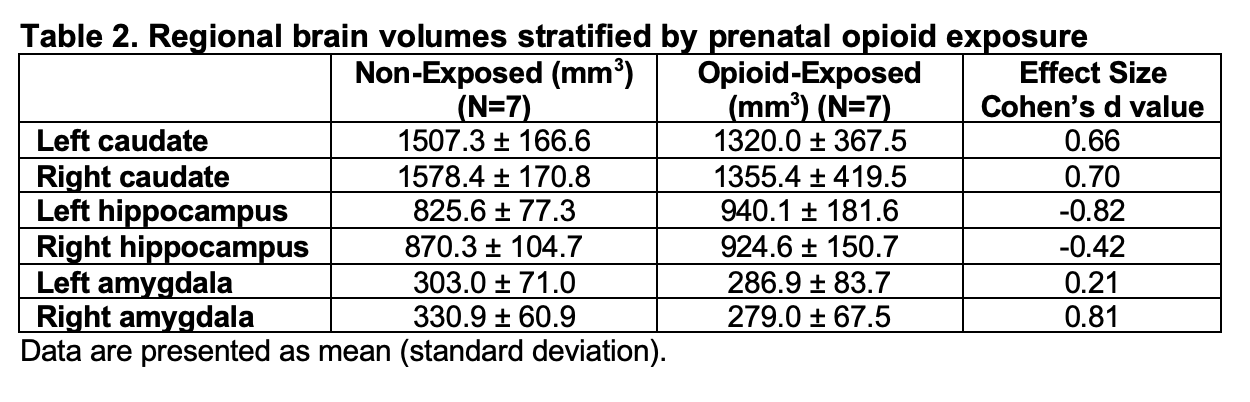
Fourteen neonatal magnetic resonance imaging (MRIs) were analyzed thus far. Demographic data showed that the two cohorts were comparable, except for the significantly lower birthweight in the exposed neonates (Table 1). Neonates with prenatal opioid exposure tended to have smaller caudates and right amygdala compared to non-exposed neonates. The effect sizes ranged from moderate to large (0.66-0.81). Hippocampus was larger in the exposed than in non-exposed neonates (Table 2).
Conclusion(s):
This pilot study indicated that opioid-exposed neonates had altered brain volumes in the regions of reward circuitry compared to non-exposed neonates. The moderate to large effect sizes suggested the strength of the prenatal opioid exposure effects on these regional brain volume differences. Our ongoing study focuses on the association between salivary reward gene expression, brain volumetric analyses, and neonatal feeding behavior.