Neonatal Clinical Trials
Neonatal Clinical Trials 1
235 - Apnea after 2-month Vaccinations in Hospitalized Preterm Infants: A Randomized Clinical Trial
Publication Number: 235.127
- RG
Rachel G. Greenberg, MD, MB, MHS (she/her/hers)
Associate Professor
Duke Clinical Research Institute
Durham, North Carolina, United States
Presenting Author(s)
Background:
The Advisory Committee on Immunization Practices recommends that preterm infants receive most vaccinations at the same postnatal age as term infants. Studies have inconsistently observed an increased risk for post-vaccination apnea in preterm infants; a perceived apnea risk may contribute to vaccination delay.
Objective:
To compare the proportions of hospitalized preterm infants with apnea and other adverse events in the 48 hours after 2-month vaccinations versus after no vaccinations.
Design/Methods:
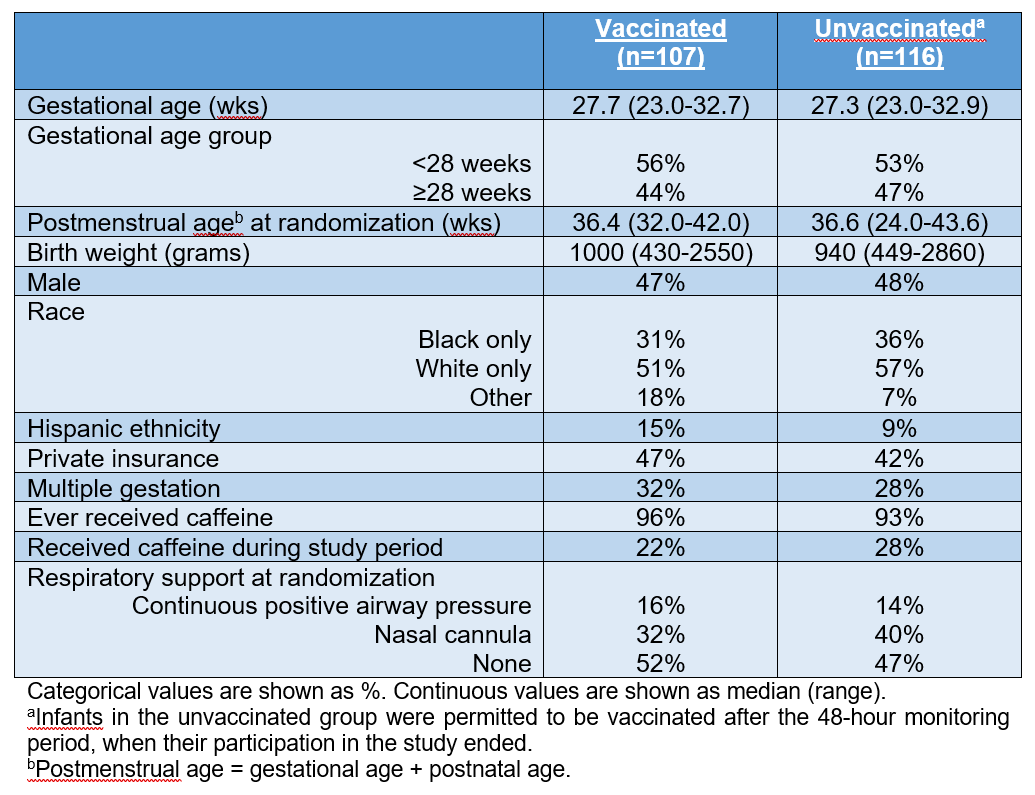
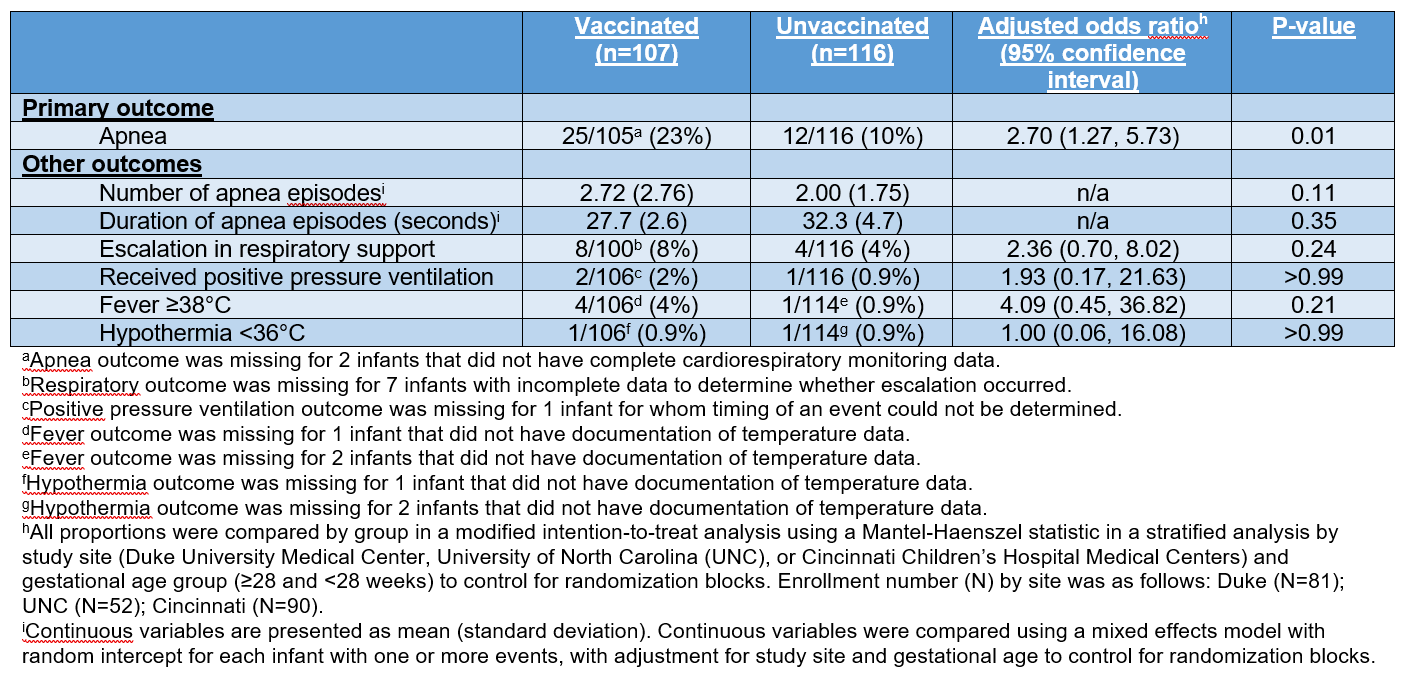
This 3-site, randomized, open-label clinical trial (NCT03530124) included hospitalized infants < 33 weeks gestational age (GA) and ≥6 and ≤12 weeks postnatal age. Infants deemed eligible to receive 2-month vaccines (Table 1) by the treating clinician were randomized 1:1 to “vaccinated” (received vaccines within 12 hours of randomization) or “unvaccinated” (received no vaccines until after study period) groups. Cardiorespiratory monitor data were collected during 48 hours after vaccination or randomization (unvaccinated group). The primary outcome was apnea, defined as a pause in respirations of >20 seconds or a pause in respirations of >15 seconds with associated bradycardia to < 80 beats per minute. Monitor-triggered events were reviewed by 2 blinded neonatologists to confirm the presence of apnea. Other outcomes included the number and duration of apnea episodes, serious adverse events (SAEs), escalation in respiratory support, receipt of positive pressure ventilation, fever ≥38°C, and hypothermia < 36°C.
Results:
223 infants were randomized: 107 vaccinated and 116 unvaccinated (Table 2). The proportion of infants with ≥1 apnea event was 23% in the vaccinated and 10% in the unvaccinated groups (adjusted odds ratio 2.70, 95% confidence interval 1.27-5.73, p=0.01). There was no difference in the mean number of apneic episodes between the vaccinated (2.72 ± 2.76) and unvaccinated (2.00 ± 1.75) groups (p=0.11). Similarly, there was no difference in the mean duration (seconds) of apneic episodes between the vaccinated (27.7 ± 2.6) and unvaccinated (32.3 ± 4.7) groups (p=0.35). No SAEs occurred during the 48-hour study period. The proportions of other outcomes were not statistically different between the vaccinated and unvaccinated groups (Table 3).
Conclusion(s):
In hospitalized preterm infants, the odds of apnea were 2.7 times higher in the 48 hours after 2-month vaccinations, but the similar number and duration of apneic events and lack of SAEs suggest current infant vaccination recommendations are appropriate. We encourage provision of evidence-based anticipatory guidance about post-vaccination apnea risk.