Neonatal Neurology: Clinical Research
Neonatal Neurology 3: Clinical 3
175 - Dexmedetomidine During Therapeutic Hypothermia Results in Improved Feeding Compared to Morphine or No Sedation
Publication Number: 175.137

Eric S. Peeples, MD, PhD (he/him/his)
Associate Professor
University of Nebraska Medical Center
Omaha, Nebraska, United States
Presenting Author(s)
Background:
Therapeutic hypothermia is standard management for infants in high income countries suffering from hypoxic-ischemic encephalopathy (HIE). The use and type of sedation during hypothermia is currently controversial, though pre-clinical and early clinical data suggest that dexmedetomidine (Dex) may decrease shivering and provide some neuroprotection. Additionally, unlike other drugs used such as opioids, Dex does not decrease gastrointestinal motility and earlier enteral feeds may decrease inflammation in infants with HIE.
Objective:
To evaluate the hemodynamic and early feeding effects of a clinical change in sedation management during therapeutic hypothermia from morphine to Dex.
Design/Methods:
This was a study of infants born at ≥35 weeks of gestation and diagnosed with moderate or severe HIE. Neonates who did not receive hypothermia or who were placed on extracorporeal membrane oxygenation in the first 12 hours of life were excluded. Based on recent data, our NICU decided to transition from the standard use of morphine to Dex for all infants receiving hypothermia. Dosing suggestions were provided, but ultimately the decisions of drug initiation and titration were driven by the clinical team. In addition to demographic data, we recorded timing and dose of Dex or opioid administration. Heart rate, blood pressure, and Neonatal Pain, Agitation, and Sedation Scale (NPASS) scores were recorded for the first twelve hours after initiation of Dex or opioid in the drug treatment groups or after admission in the no treatment group.
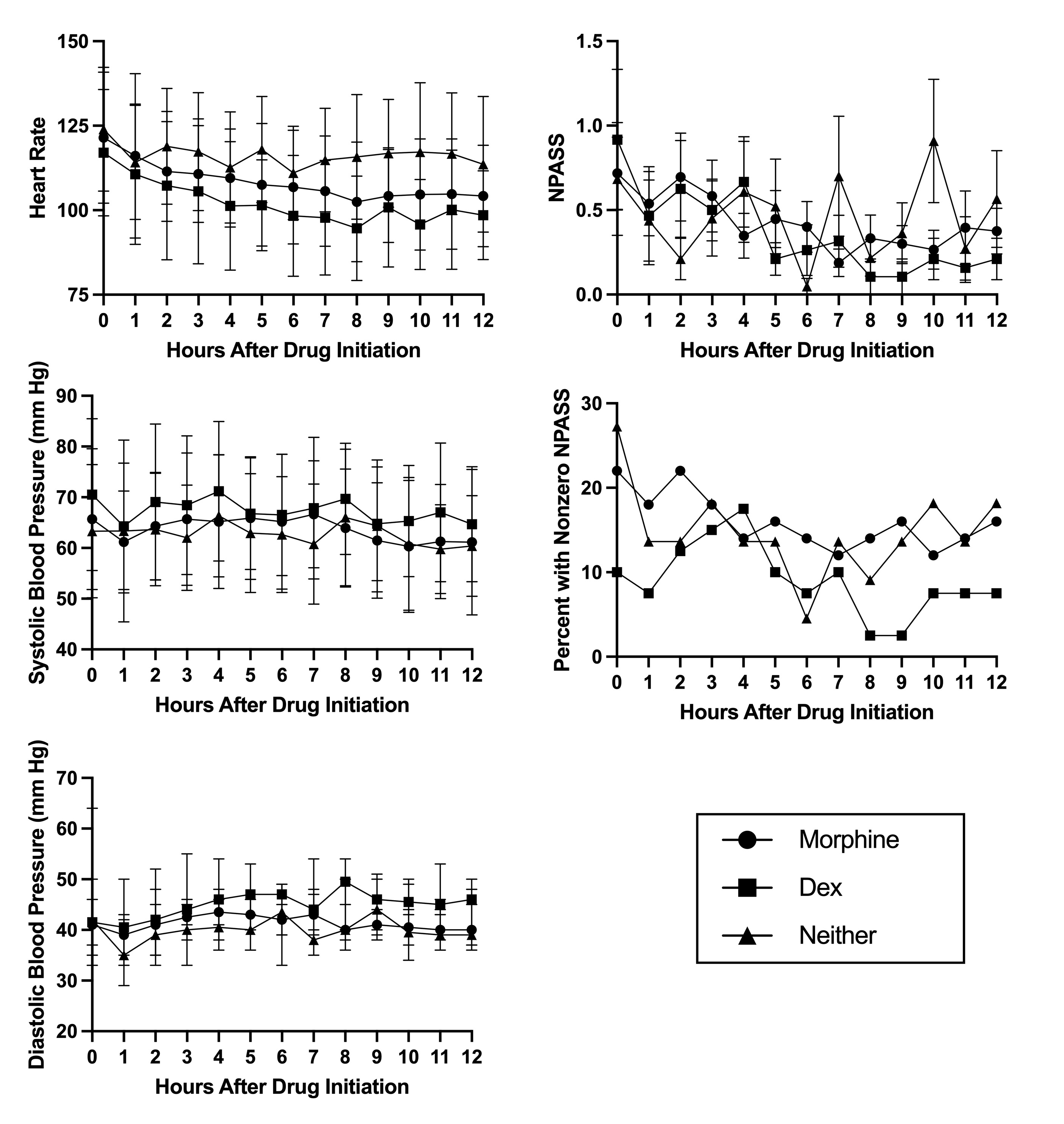
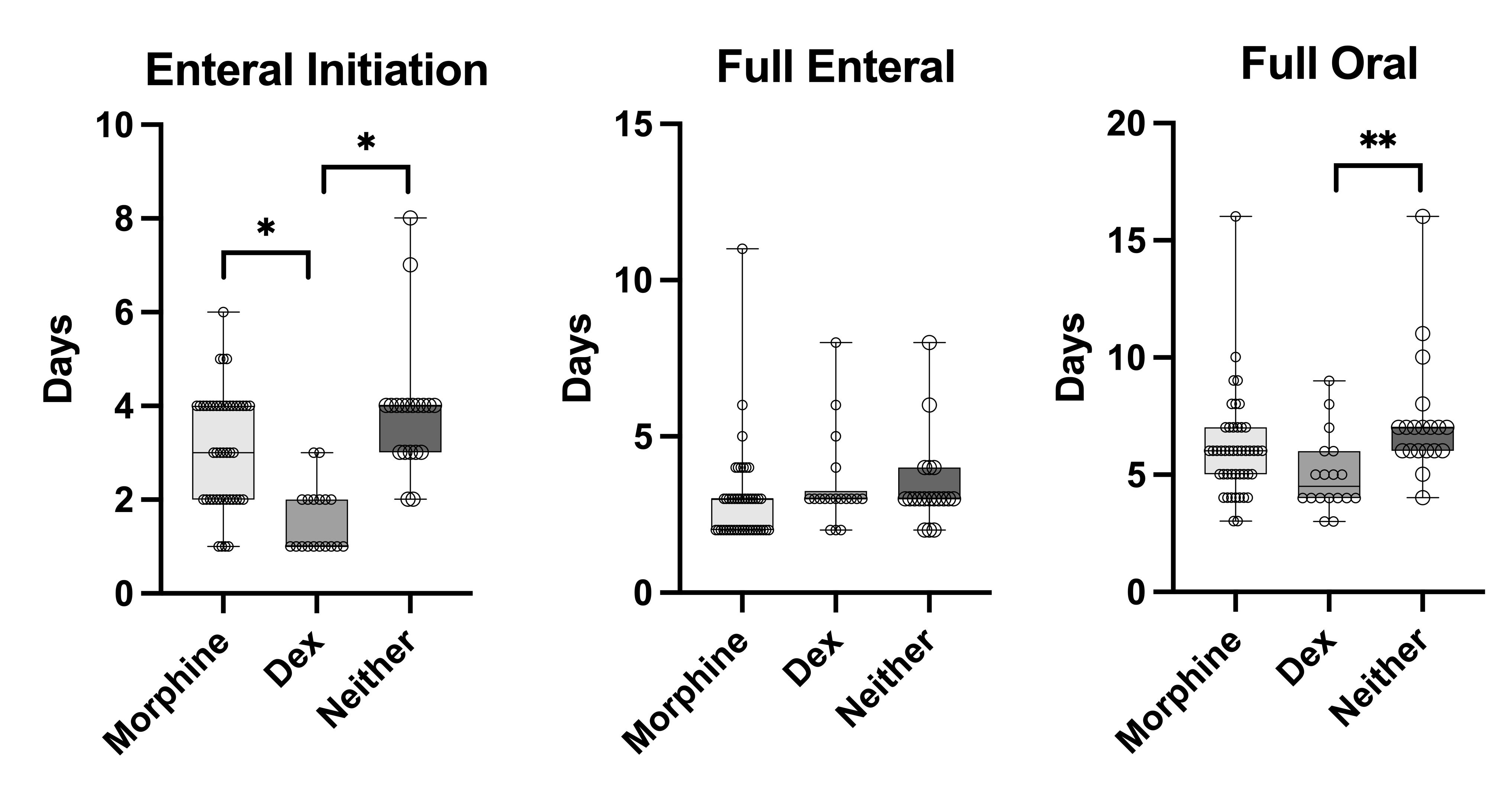
Results: Ninety-one infants were included: 44 receiving morphine (2.5-20 mcg/kg/hr), 19 Dex (0.1-0.4 mcg/kg/hr), and 28 neither. There were no significant differences in heart rate, blood pressure, or NPASS scores between groups. The percent of infants with non-zero NPASS scores tended to be lowest in the Dex group at >7 hours after drug initiation (Fig.1). Infants receiving Dex initiated enteral feeds earlier than either of the other groups, though there was no difference in the days to reach full enteral feeds between groups (Fig.2). The Dex group also reached full oral feeds earlier than the no treatment group but no different than the morphine group.
Conclusion(s):
Dex had no significant adverse effects on heart rate or blood pressure and may result in less non-zero N-PASS scores compared to morphine or no treatment. The differences in timing of initiation of feeds is more likely due to recent increases in clinician comfort with feeding during hypothermia, though we did see a decrease in time to full oral feeds in the Dex group compared to no treatment.