Emergency Medicine: All Areas
Emergency Medicine 2
32 - Dexamethasone Dosing for Pediatric Acute Asthma Exacerbations: Prescribing Practices and Outcomes
Friday, April 28, 2023
5:15 PM - 7:15 PM ET
Poster Number: 32
Publication Number: 32.111
Publication Number: 32.111
Melisa S.. Tanverdi, University of Colorado School of Medicine, Denver, CO, United States; Nidhya Navanandan, University of Colorado School of Medicine, Aurora, CO, United States; Savannah Brackman, University of Colorado School of Medicine, Denver, CO, United States; Lorel Huber, University of Colorado School of Medicine, Aurora, CO, United States; Jan Leonard, University of Colorado School of Medicine, Aurora, CO, United States; Rakesh D.. Mistry, Children's Hospital Colorado, Aurora, CO, United States

Melisa S. Tanverdi, MD (she/her/hers)
Assistant Professor
University of Colorado School of Medicine
University of Colorado School of Medicine
Aurora, Colorado, United States
Presenting Author(s)
Background: Dexamethasone is increasingly used as the preferred corticosteroid for acute asthma in children. However, current emergency department (ED) dexamethasone prescribing practices and clinical outcomes are not known.
Objective: To evaluate dexamethasone prescribing practices, patient adherence, and clinical and patient-focused outcomes by dosing regimen (one versus two doses) in children with acute asthma exacerbations discharged from the ED.
Design/Methods: Prospective study of children 2-18 years treated in a pediatric ED for an acute asthma exacerbation that received a dose of oral dexamethasone prior to discharge. The primary outcome was treatment failure, defined as return ED visit, unplanned primary care visit, and/or ongoing bronchodilator use at 7-10 days, and was evaluated via telephone follow-up. Secondary outcomes were also assessed 7-10 days post-ED visit and included medication adherence, symptom persistence and quality of life (QOL), as measured by validated scales, and school/work absenteeism. Demographics, clinical characteristics, and dexamethasone prescribing practices at ED visit were collected via medical record review. Multivariable logistic regression was used to assess the association between dexamethasone dosing regimen and treatment failure.
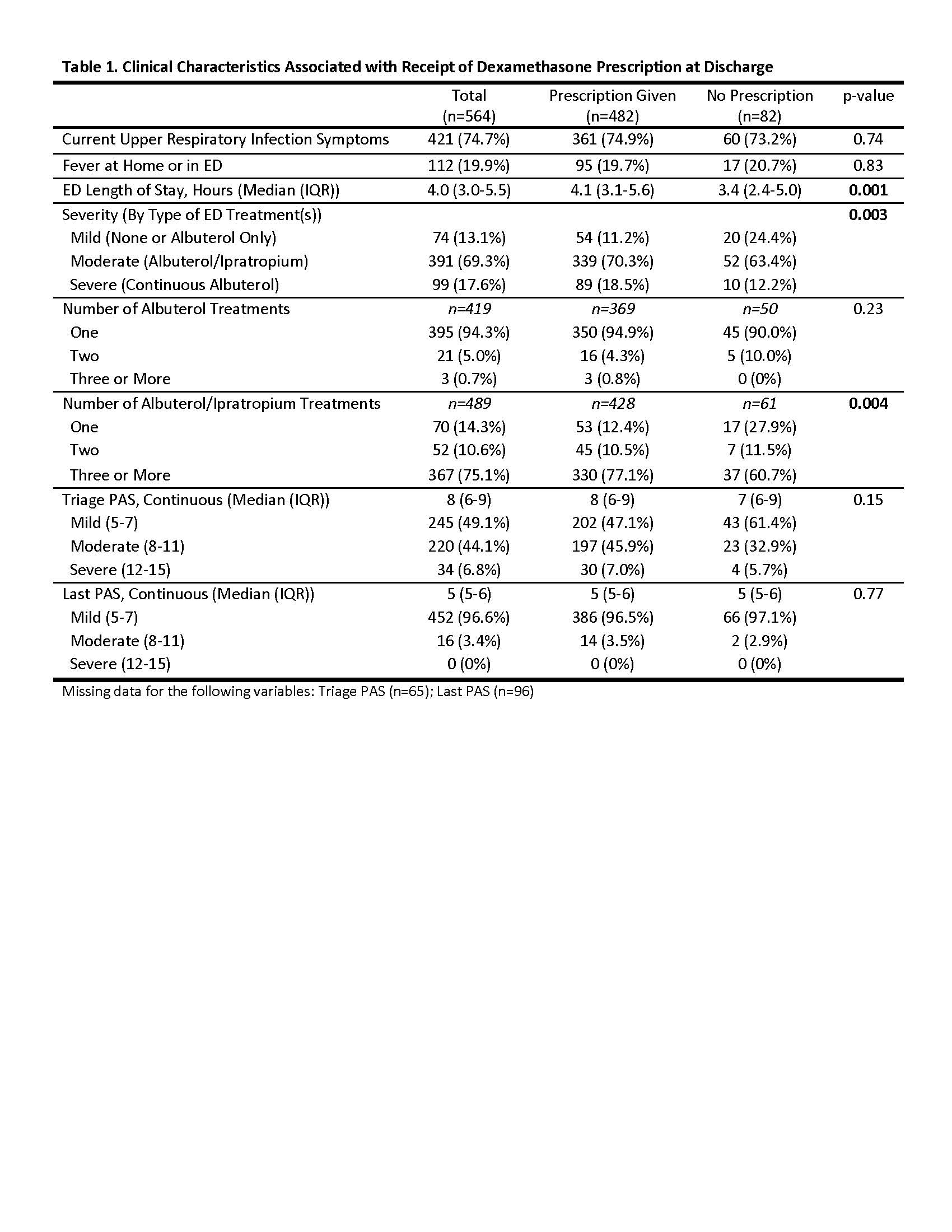
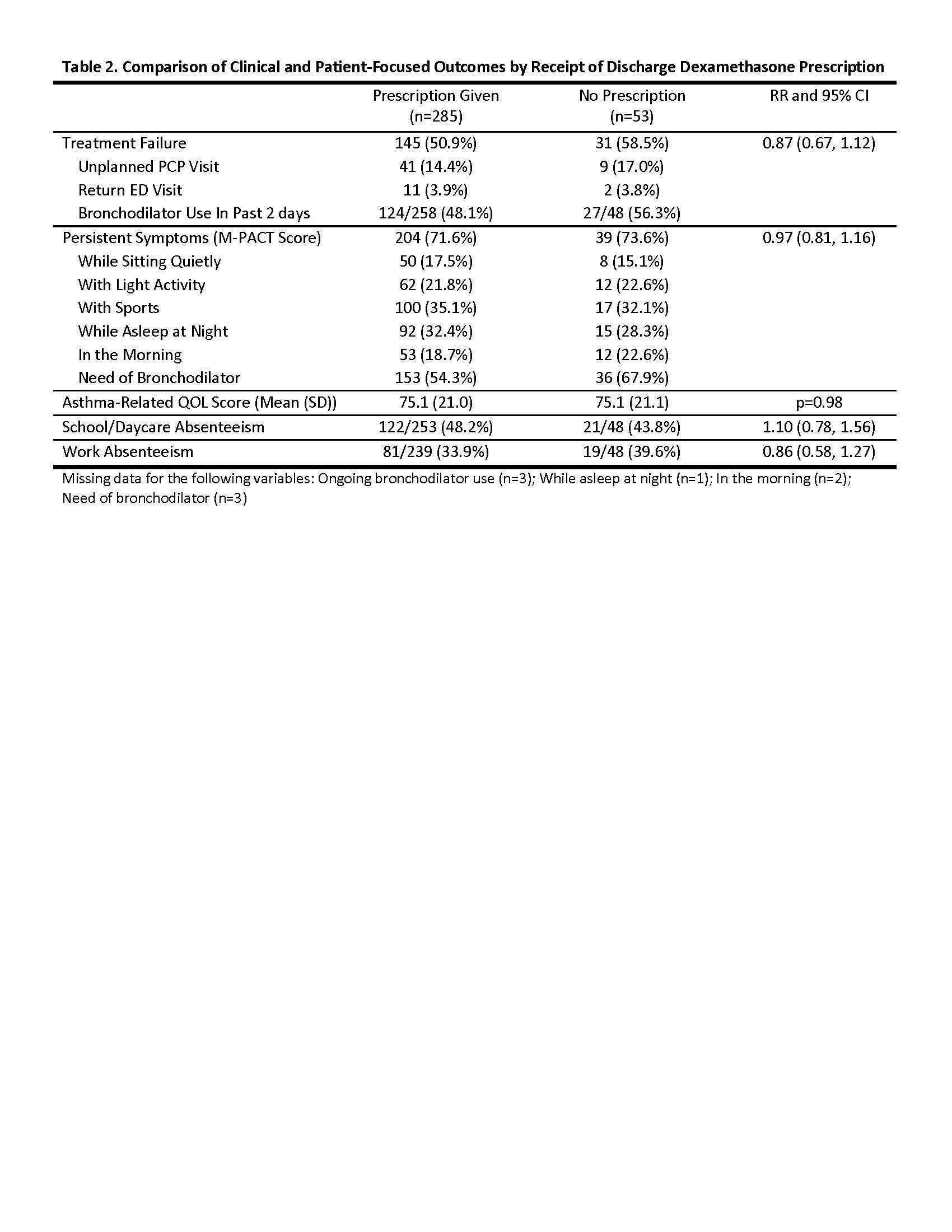
Results: 564 subjects were enrolled; 338 caregivers (60.0%) completed follow-up. Children had a median age of 7 years, 30% were African American, 49% were Hispanic, and 79% had public insurance. A discharge prescription for an additional dose of dexamethasone was written for 482 (86%) children. The median dose prescribed was 0.5 mg/kg (0.4-0.6) with a maximum of 16 mg. A discharge prescription for dexamethasone was significantly associated with severity of acute exacerbation, number of combined albuterol/ipratropium treatments, and longer ED length of stay (Table 1). Of caregivers who reported receipt of a discharge prescription for dexamethasone, most (89%) reported filling and administering the dose. There was no difference in treatment failure between those who received a dexamethasone prescription and those who did not (RR 0.87; 0.67, 1.12) in multivariable analysis; there was also no difference in secondary outcomes of symptom persistence, QOL, or school/work absenteeism between the two groups (Table 2).
Conclusion(s): Prescription for an additional dose of dexamethasone was not associated with reduced treatment failure or improved clinical or patient-focused outcomes for children with acute asthma discharged from the ED. Single dose of dexamethasone in the ED may be sufficient for treatment of children presenting for acute exacerbations.