Mental Health
Mental Health 1
577 - Differential Disclosure in Depression Screening in Pediatric Clinical Care versus Research
Publication Number: 577.126
- BC
Barbara H. Chaiyachati, MD/PhD (she/her/hers)
Assistant Professor
Children's Hospital of Philadelphia, University of Pennsylvania
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background:
Maternal depression screening is recommended as part of routine pediatric primary care by the American Academy of Pediatrics. Reliability of mental health symptom disclosure across research and clinical settings has been rarely quantified in pragmatic, contemporary settings.
Objective:
Determine the reliability of maternal depression screening by Edinburgh postpartum depression scale (EPDS) across research and clinical settings.
Design/Methods:
This was a secondary analysis of a subset of a longitudinal observational cohort (n=913) study of pregnancy and child development in Philadelphia. As part of the overarching study, participants completed a postpartum EPDS (n=833, “research EPDS”). Of those, 183 also had EPDS available as part of pediatric healthcare (“clinical EPDS”) within any 4-week window of their research assessment. Item 10 (endorsement of self-harm) was excluded from analysis.
To analyze the reliability of EPDS scores across the two settings, we examined intraclass correlations (ICC) of mean EPDS scores, mean differences in EPDS scores using a two tailed t-test and two tailed Pearson’s correlations. We also examined differences in the number of positive screens by threshold (score of ≥10).
Results:
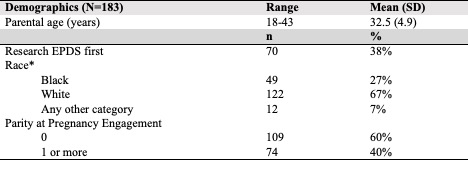
Mean maternal age was 32.5 ± 4.9 years and 38.3% (n=70) participants completed the research EPDS first (Table 1).
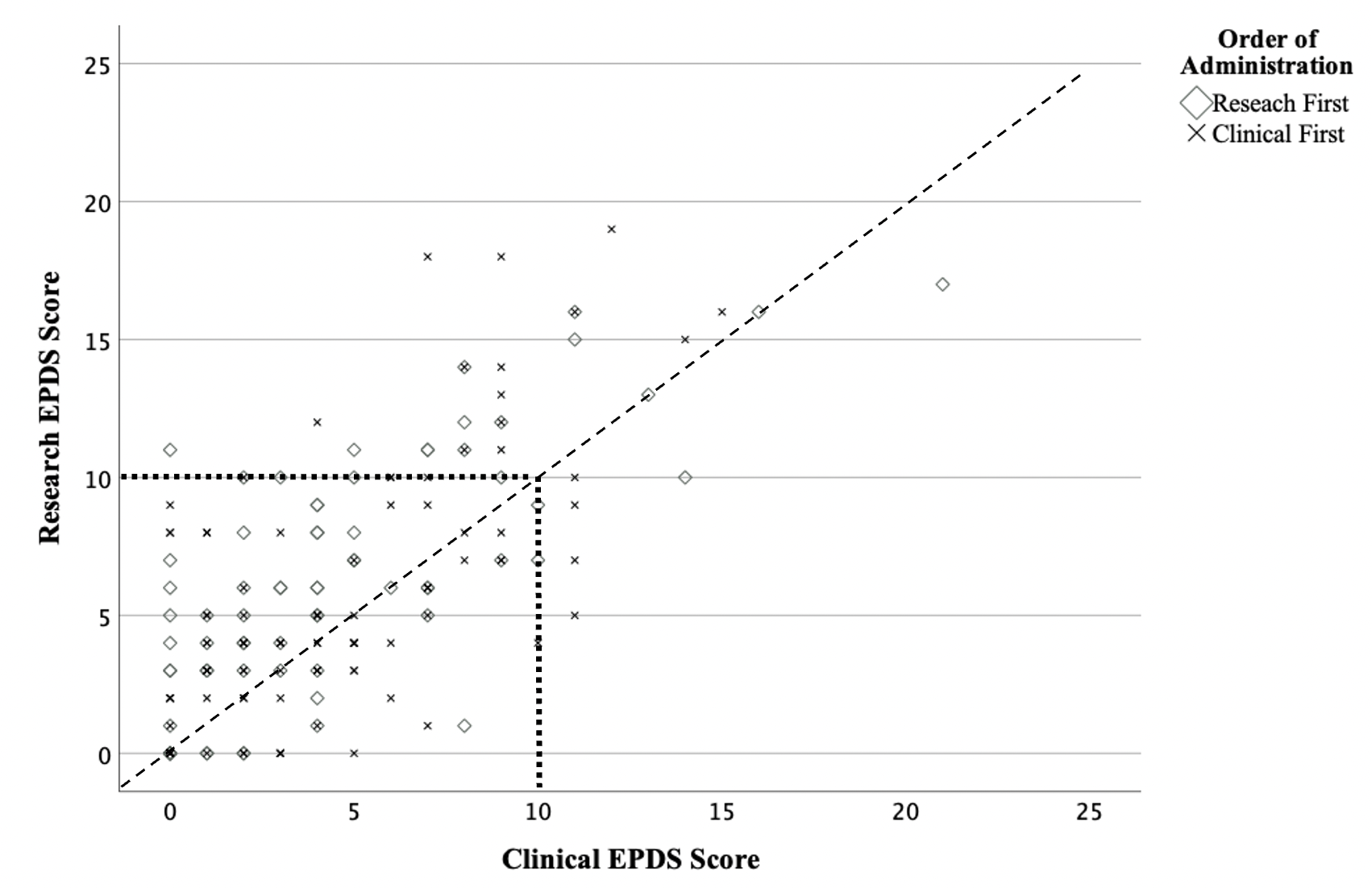
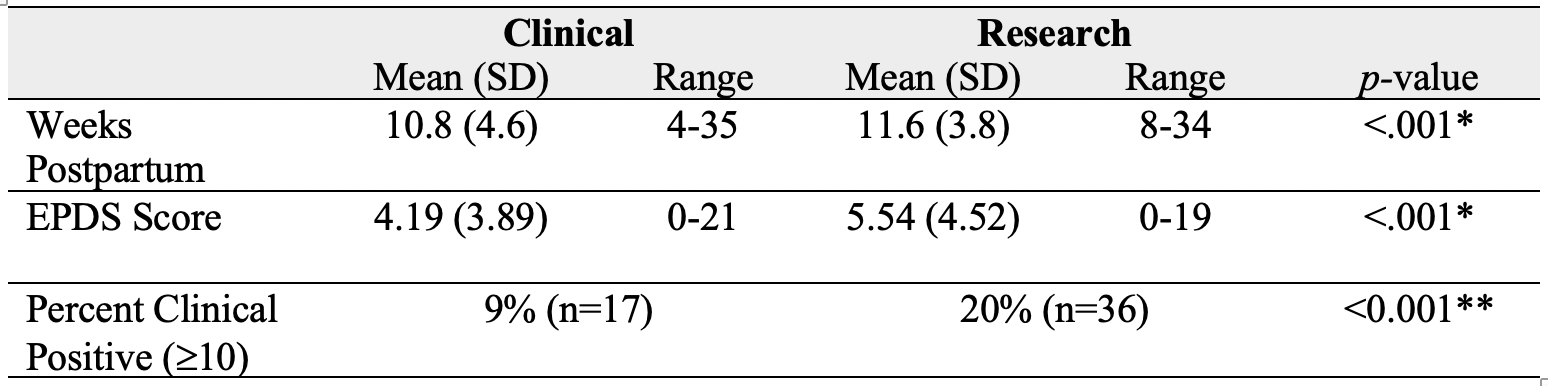
While the ICC (.83) and correlation (r(183)=.72, p< .001) of total scores across settings were high, the mean total research EPDS score in the research setting (5.54 ± 4.52) was significantly higher than the total score in the clinic setting (4.19 ± 3.89, p< .001; Table 2; Figure 1).
Using a threshold score, 36 individuals (20% of the sample) screened positive by the research EPDS while only 17 (9% of the sample) screened positive by the clinical EPDS (p< 0.001). Of the 42 that screened positive in either setting, 11 individuals met threshold at both settings, 25 met threshold from the research EPDS but did not meet threshold from the clinical EPDS, and 6 individuals screened positive at the clinical EPDS but not research.
Conclusion(s):
Mothers of young infants disclosed more symptoms and were more likely to screen positive for depression symptoms per threshold by EPDS in the research than the clinical setting. Drivers of differential disclosure may include desirability bias, concerns of stigma, privacy, and other perceived ramifications of disclosure to pediatricians. Underreporting in clinical settings may undermine recognition of need with potential for systematic patterns.