Neonatal Fetal Nutrition & Metabolism
Neonatal Fetal Nutrition & Metabolism 2: Neonatal Nutrition, Growth, and Outcomes
311 - Prolonged Riboflavin Deficient Parenteral Nutrition Exposure and the Risk of Inborn Error of Metabolism Mimic
Publication Number: 311.129

Rachel Graf, BS (she/her/hers)
Medical Student
Ohio State University College of Medicine
Columbus, Ohio, United States
Presenting Author(s)
Background:
Beginning January 2021, manufacturing delays created a shortage of commercial formulation of IV pediatric multivitamins used in parenteral nutrition (PN). Pharmacies resorted to compounding PN with individually formulated vitamins and riboflavin was unavailable. Infants receiving PN were at risk of developing riboflavin deficiency. As riboflavin is a cofactor in biochemical pathways, riboflavin deficiency can mimic metabolic disorders in neonates.
Objective:
To determine the incidence of vitamin B2 deficiency in infants who received prolonged (≥14 consecutive days) riboflavin deficient PN in the Nationwide Children’s Neonatal Network.
Design/Methods:
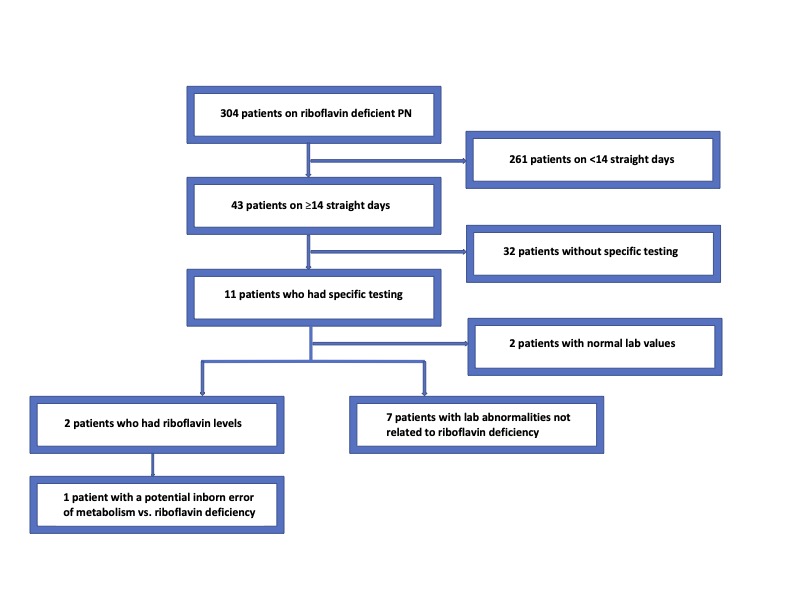
Demographic and biochemical data on all patients receiving riboflavin deficient PN from January to August 2021 was abstracted from the medical record. 43 neonates received prolonged riboflavin deficient PN and were further reviewed.
Results:
The median length of riboflavin deficient PN was 24 days with an interquartile range (IQR) of 17-33. Subjects were 44% female, born at a median gestational age (GA) of 26 weeks (IQR: 24-34) and a median birthweight of 850 g (IQR: 600-1868). The median age of starting PN was 10 days (IQR: 4-46). 18.6% (8/43) subjects died prior to discharge.
11/43 (25.6%) subjects had specific biochemical testing while receiving riboflavin deficient PN. 18% (2/11) had riboflavin deficiency (<4 µg/dL). One of these two had an acylcarnitine profile (ACP) consistent with multiple acyl-CoA dehydrogenase deficiency. The other patient had an undetectable riboflavin level, but the ACP was not consistent with an inborn error of metabolism. The patient later died. Most subjects’ abnormal tests (5/11, 56%) were due to PN or carnitine supplementation. Carnitine uptake deficiency, 3-methylglutaconic-CoA hydratase deficiency and a benign disorder of histidine metabolism were incidentally noted in one subject each.
Conclusion(s):
The mortality rate is comparable to neonates born at 29 weeks GA. Most abnormal biochemical testing was attributable to routine care. One subject had labs that required consideration of an inborn error of metabolism. This subject did well after riboflavin reintroduction.
As both patients tested for riboflavin were deficient, other patients were potentially deficient but undetected. During future shortages, prospective surveillance should be instituted. Neonatal providers should be aware that prolonged deprivation of essential cofactors can cause biochemical abnormalities that mimic inborn errors of metabolism. If studies cannot be obtained, consideration should be given to empiric provision of cofactors in sick neonates.