Neonatal Hematology & Bilirubin Metabolism
Neonatal Hematology & Bilirubin Metabolism 2: Hematology
128 - Sex-differences in hematopoietic stem and progenitor cell function and transcriptome in offspring of obese mice
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 128
Publication Number: 128.239
Publication Number: 128.239
Merve Denizli, Indiana University School of Medicine, Indianapolis, IN, United States; James Ropa, Indiana University School of Medicine, Indianapolis, IN, United States; Lindsay Beasley, Indiana University School of Medicine, Indianapolis, IN, United States; Laura Haneline, Indiana University School of Medicine, Indianapolis, IN, United States; Maegan Capitano, Indiana University School of Medicine, Indianapolis, IN, United States; Kok Lim Kua, Indiana University School of Medicine, Indianapolis, IN, United States
.jpg)
Merve Denizli, MD (she/her/hers)
Neonatal-Perinatal Fellow
Indiana University School of Medicine
Indianapolis, Indiana, United States
Presenting Author(s)
Background: Maternal obesity is increasingly common and can negatively impact offspring health. Children born to mothers with obesity are at higher risk of developing diseases such as obesity, type 2 diabetes, allergy/asthma, and leukemia. Many of these diseases have altered immune profiles or hematopoiesis with sex-differences in risks, suggesting the sex-specific reprogramming of hematopoietic stem and progenitor cells (HSPC). However, the sex-differences in offspring HSPC function and the related mechanisms have not been studied.
Objective: This study aims to test the hypothesis that offspring of obese mice exhibit sex-differences in HSPC function with associated transcriptome changes.
Design/Methods: Female dams were fed with chow (Con) or Western diet (MatOb) for four weeks prior to mating, through pregnancy, and the lactating period. At postnatal day 21, HSPC (Lin- Sca-1+ cKit+ cells) were flow-sorted from offspring bone marrow for competitive primary and secondary transplant to assess HSPC function. RNA-seq was performed on HSPC and differentially expressed genes (DEG) were determined using DESeq2 (adjusted p-value < 0.05) and subjected to pathway analysis using IPA (adjusted p-value< 0.05, z-score ≤ or ≥ 0.5).
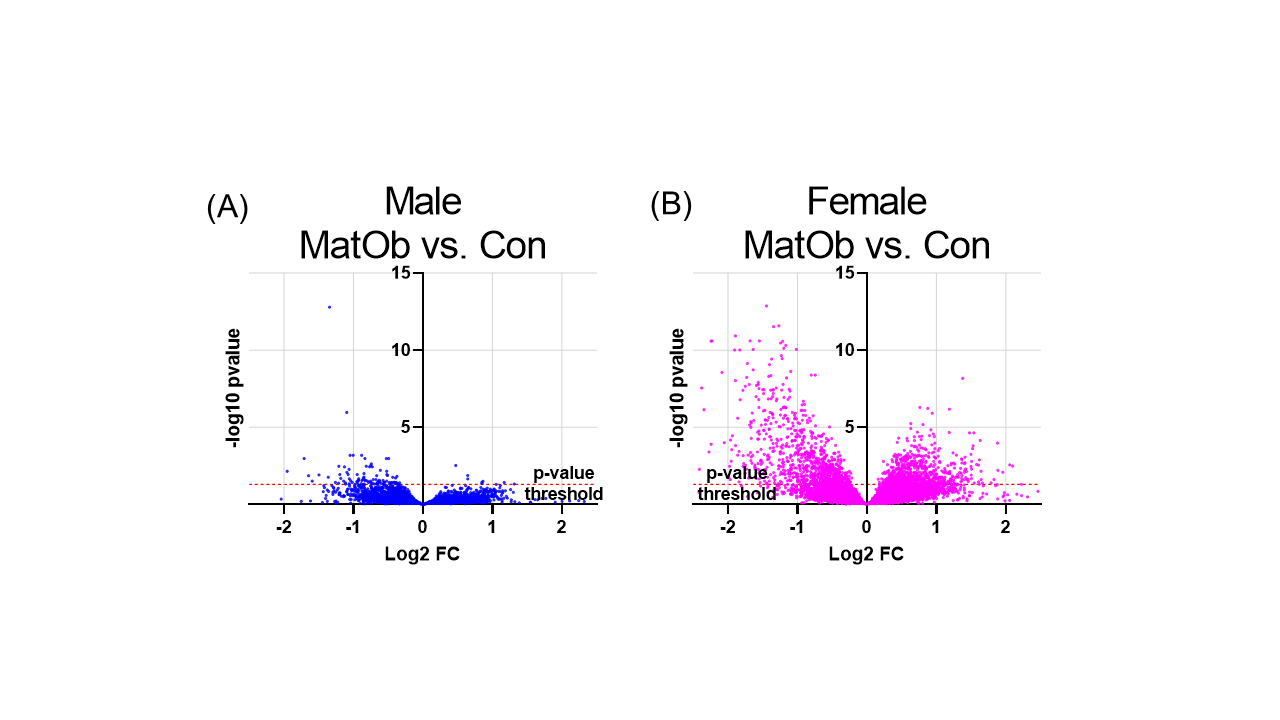
Results: Compared to same-sex Con, HSPC from male MatOb had higher donor chimerism 16 weeks following primary transplantation, while donor chimerism of HSPC from female pups was unchanged. Following the secondary transplant, recipients of male MatOb HSPC had significantly lower donor chimerism at 4 and 16 weeks, and lower donor chimerism in the bone marrow HSPC, indicating male MatOb HSPC had decreased function in response to prolonged stress (p< 0.05, n ≥5 pups from ≥3 litters/grp). Compared to same-sex Con, RNAseq revealed 100 DEG (8 up- and 92 down-regulated) in male MatOb HSPC, and 1788 DEG (823 up- and 965 down-regulated) in female MatOb HSPC (n=6/sex/group, Fig 1). Pathway analysis identified disease and function pathways related to metabolism and hematopoiesis common in both male and female MatOb HSPC. Interestingly, multiple canonical pathways were positively enriched (e.g. chemokine signaling, ERK/MAPK signaling) and “apoptosis of hematopoietic progenitor cells” was predicted to be downregulated only in female MatOb HSPC (Fig 2).
Conclusion(s): Maternal obesity results in sex-specific changes in offspring HSPC function and transcriptome. Future experiments are directed to validate potential pathways and mechanisms contributing to sex-differences in HSPC function.
.png)
