Neonatal General
Neonatal General 5: COVID, Infections Diseases
701 - Development and Implementation of guidelines for Ventilatory Associates Pneumonia in Neonates
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 701
Publication Number: 701.233
Publication Number: 701.233
Shamaila gill, University of Texas Southwestern Medical School, frisco, TX, United States; Michael Sebert, University of Texas Southwestern Medical School, Dallas, TX, United States
- Sg
Shamaila gill, MD
Assistant Professor of Pediatrics
University of Texas Southwestern Medical School
frisco, Texas, United States
Presenting Author(s)
Background: Diagnosis and treatment of VAP and VAT is challenging as there is no gold standard for diagnosis in neonates. The Clinical Pulmonary Infection Score (CPIS) has been used in adult and pediatric populations for VAP/VAT diagnosis, but there are limited data in neonatal populations
Objective: Implementation of an algorithm for management of VAP and VAT in neonates at the Children's Medical Center NICU with a target of 50% compliance or greater by December 312021.
Design/Methods:
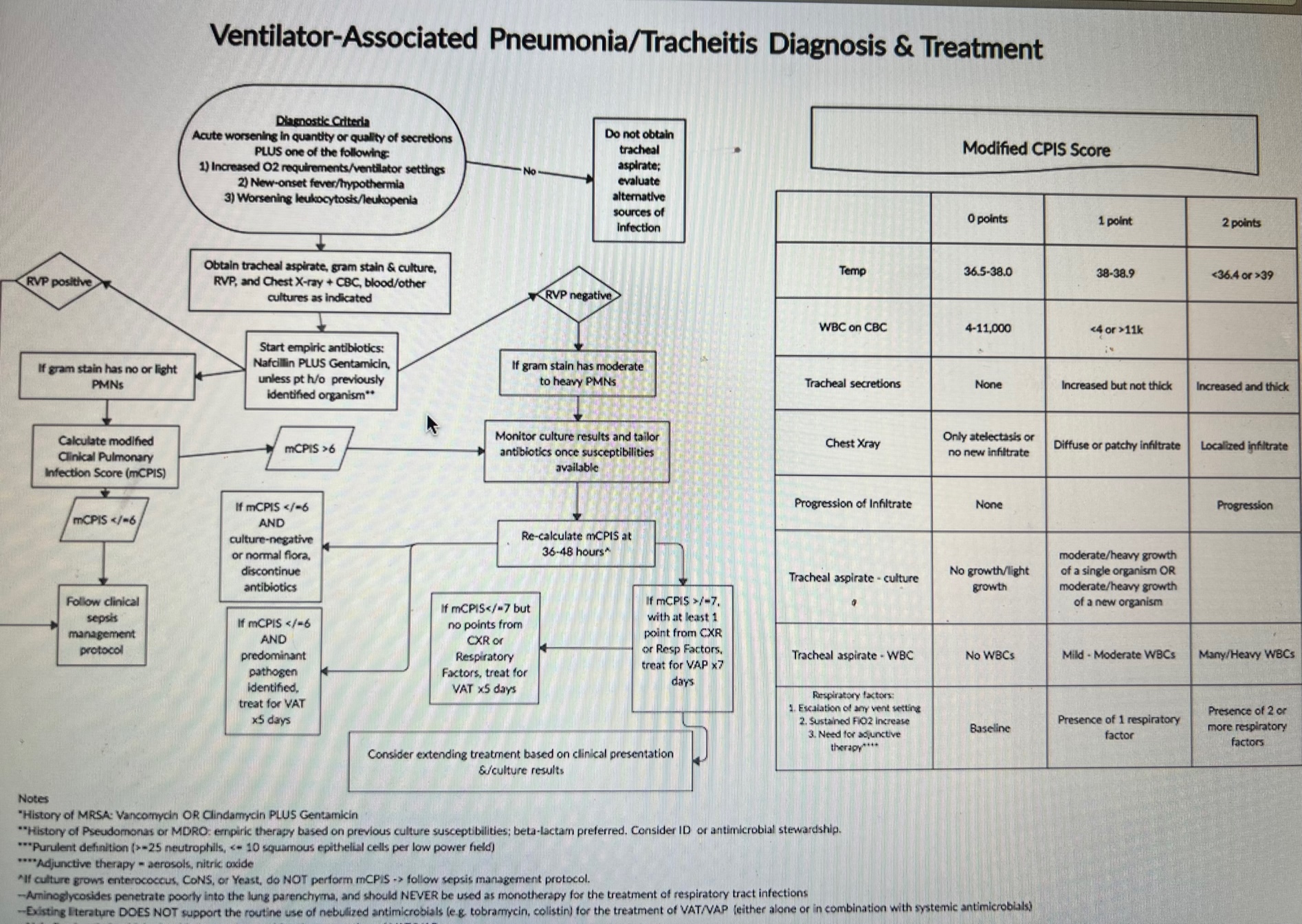
A literature review was conducted to identify published guidelines and tools for the diagnosis and treatment of VAP/VAT. A multidisciplinary team developed a VAP/VAT guideline synthesizing examples from other children’s hospitals and incorporating a modified CPIS (mCPIS) to aid in the diagnosis and treatment of VAP/VAT (Figure1). The entry criteria were based on CDC guidelines for diagnosing pneumonia in infants < 1year of age. Baseline data on VAP/VAT diagnosis and treatment prior to the implementation of the new algorithm were collected. Monthly audits to monitor guideline adherence and sensitivity and specificity of mCPIS scoring and guideline entry criteria were collected. Diagnostic criteria for VAP/VAT established for this study were as follows: (1) Tracheal aspirate with heavy to moderate growth of organism, and (2) Either no prior tracheal cultures positive for the organism, or Gram stain with moderate to heavy WBC. VAP: diagnostic criteria + mCPIS > 6 with at least 1 point from CXR or respiratory factor. VAT: diagnostic criteria + mCPIS ≤ 6 and no points from CXR or respiratory factors
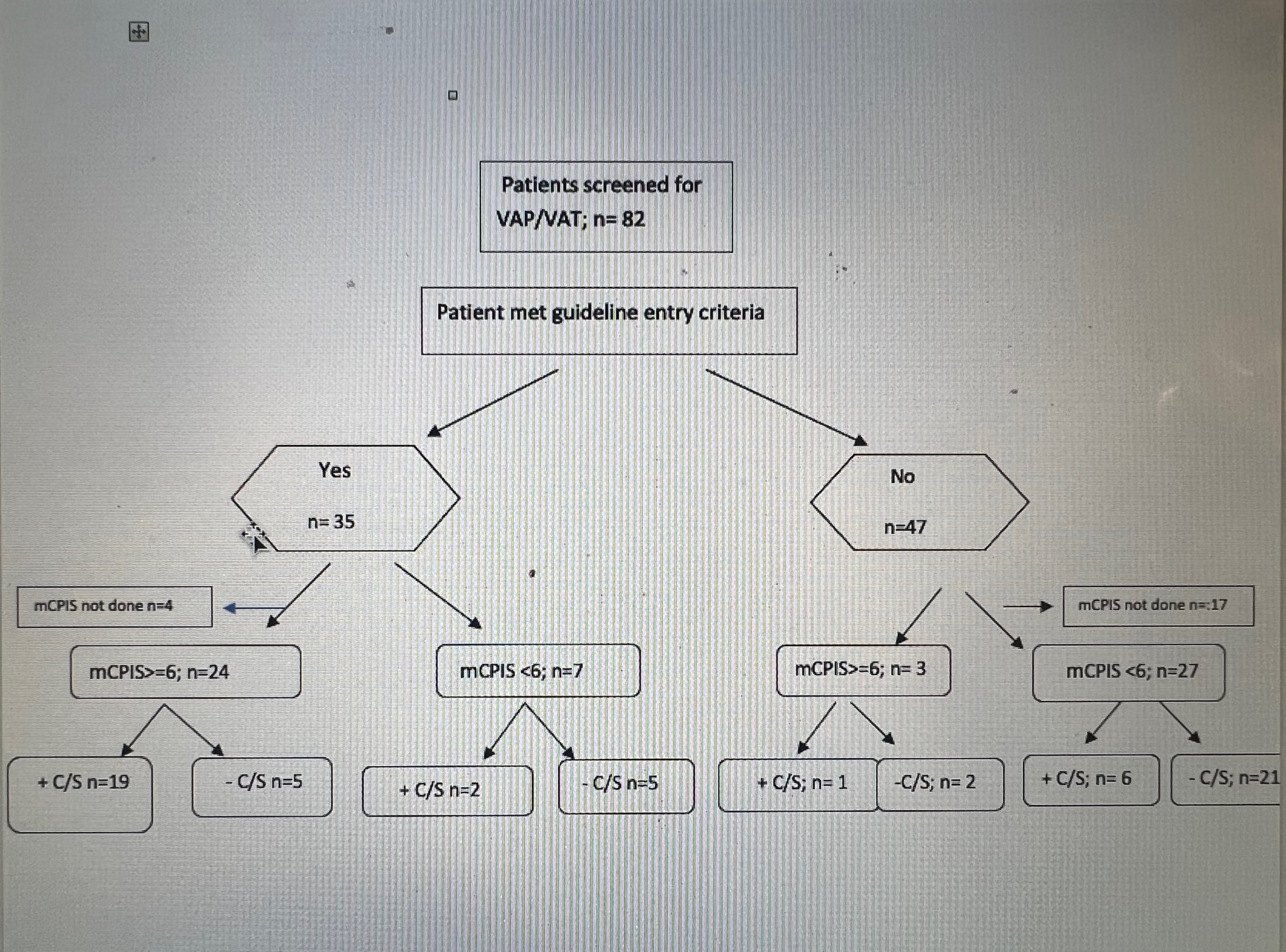
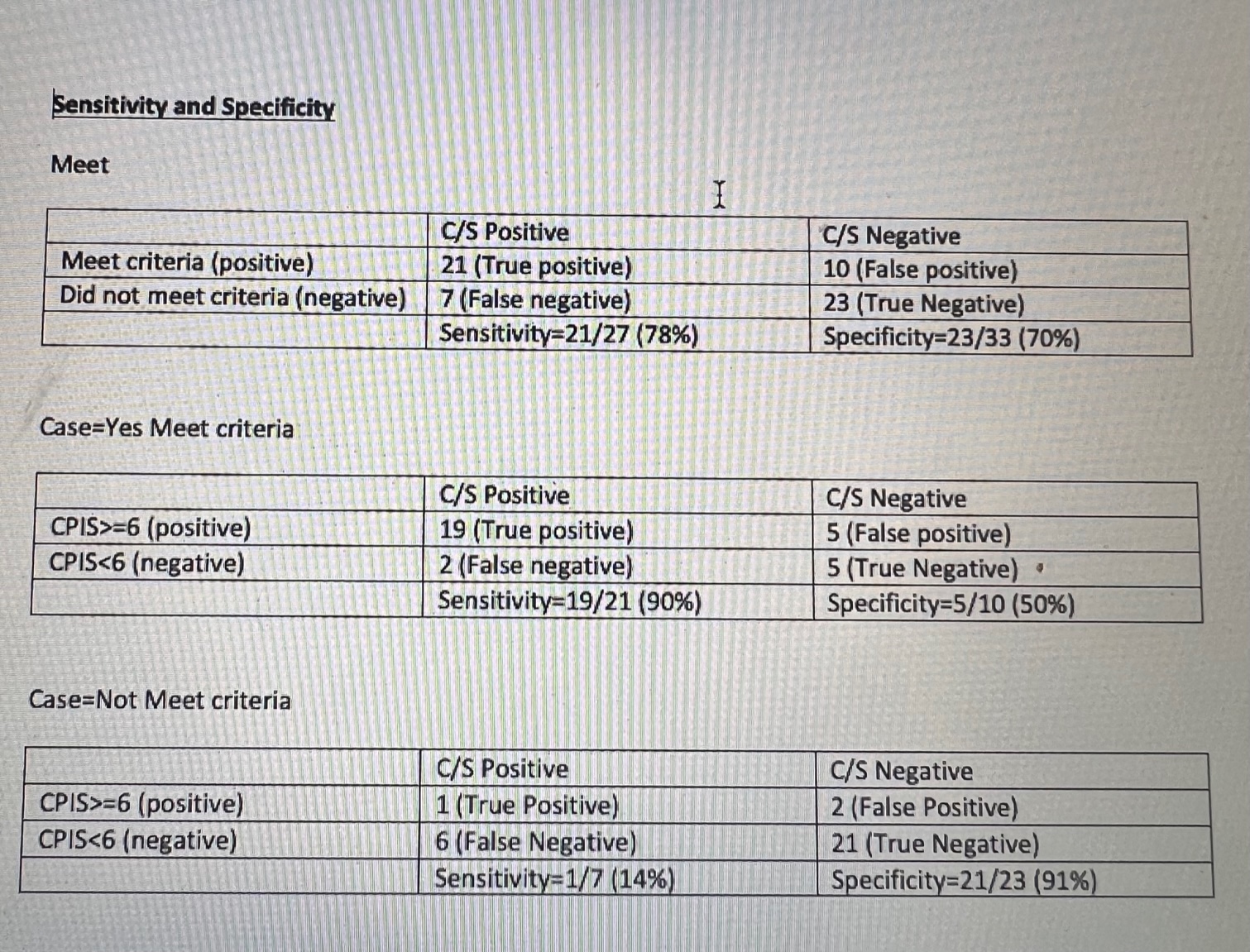
Results: Data were collected from October 2020 to May 2021(Figure 2). The baseline guideline entry criteria to initiate workup had 78% sensitivity and ~70% specificity for infection (presence of either VAP or VAT, Table 1). Of patients who met guideline entry criteria, a mCPIS ≥ 6 increased the sensitivity to 91% and specificity 50% for infection (Table 2). In contrast, among patients who did not meet entry criteria but for whom clinicians nevertheless sent tracheal aspirates, mCPIS ≥ 6 had sensitivity of only 14% but specificity of 91% (Table 3) The overall compliance rate=20/46 (43.5%) (Figure 3). The VAP/VAT diagnosis rate prior to implementation of guidelines was (60%) and post implementation rate is 50%
Conclusion(s): The CDC guideline entry criteria had a specificity ~ 78% for infection but, when combined with the mCPIS tool, sensitivity improved to ~ 90% for infection. Hence mCPIS can be utilized as diagnostic tool to diagnose VAP/VAT