Breastfeeding/Human Milk
Breastfeeding/Human Milk 3: Human Milk Bioactives and Composition
16 - SARS-CoV-2 IgG dominant response in human milk, plasma, and infant's stool after mRNA COVID-19 booster
Publication Number: 16.201

Vivian Valcarce, MD (she/her/hers)
Neonatal-Perinatal medicine fellow
University of Florida College of Medicine
Gainesville, Florida, United States
Presenting Author(s)
Background:
Human milk provides the postnatal link that promotes maternal-infant immune dialogue. Immunoglobulins, present in human milk, provide passive immunity against relevant viruses in the mother-infant environment.1 With the surge of the COVID-19 pandemic and new mRNA vaccines, efforts to study the effects of the vaccine in lactating women and breastfeeding infants were made worldwide. We now know that vaccine is safe for mother and infant, and SARS-CoV-2-specific antibodies are secreted in human milk in response to vaccination.2 Our previous findings show vaccinated mother's milk can neutralize the virus in-vitro, which could protect the infant against COVID-19 infection.3
Objective:
To study the long-lasting effect of COVID-19 vaccination and booster in human milk, plasma, and breastfeeding infants' stool.
Design/Methods:
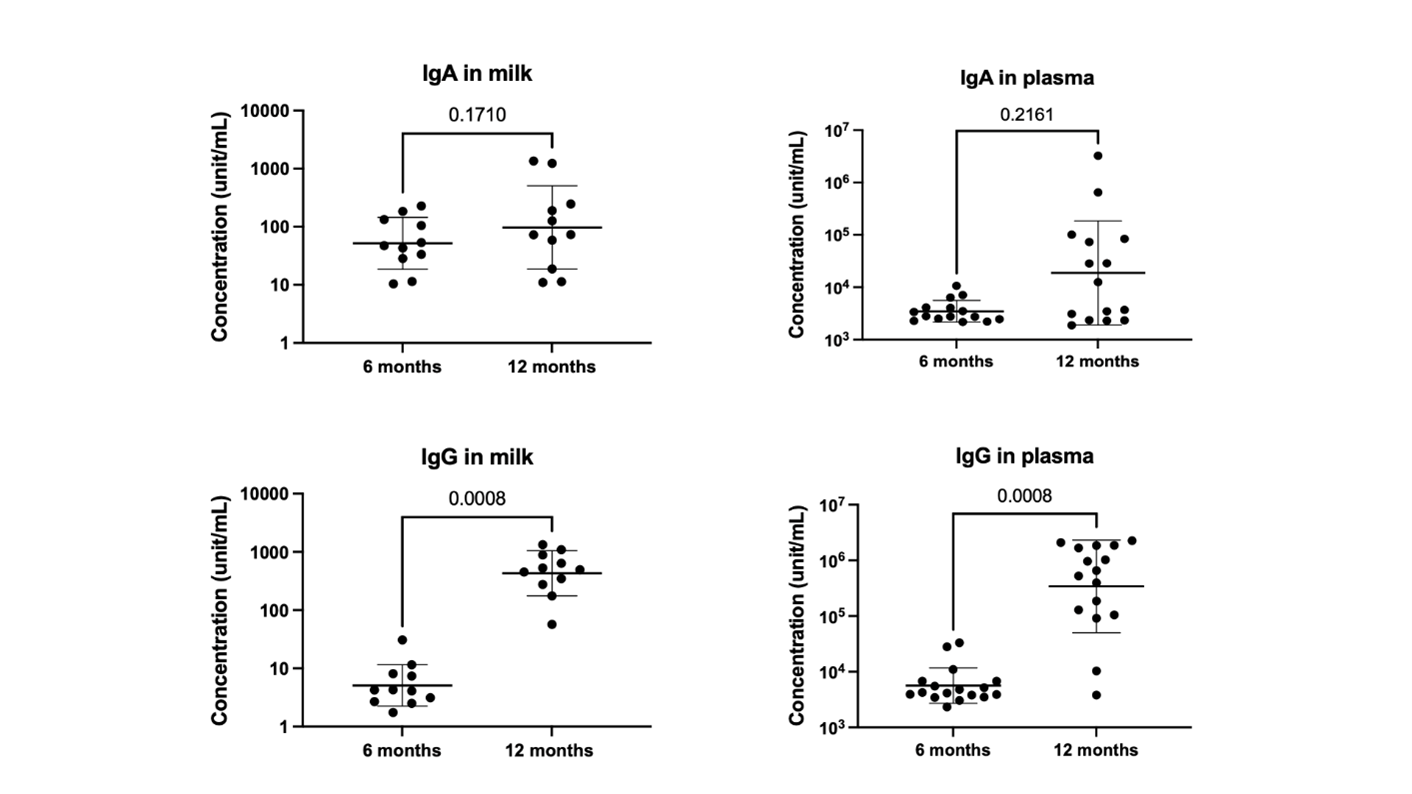
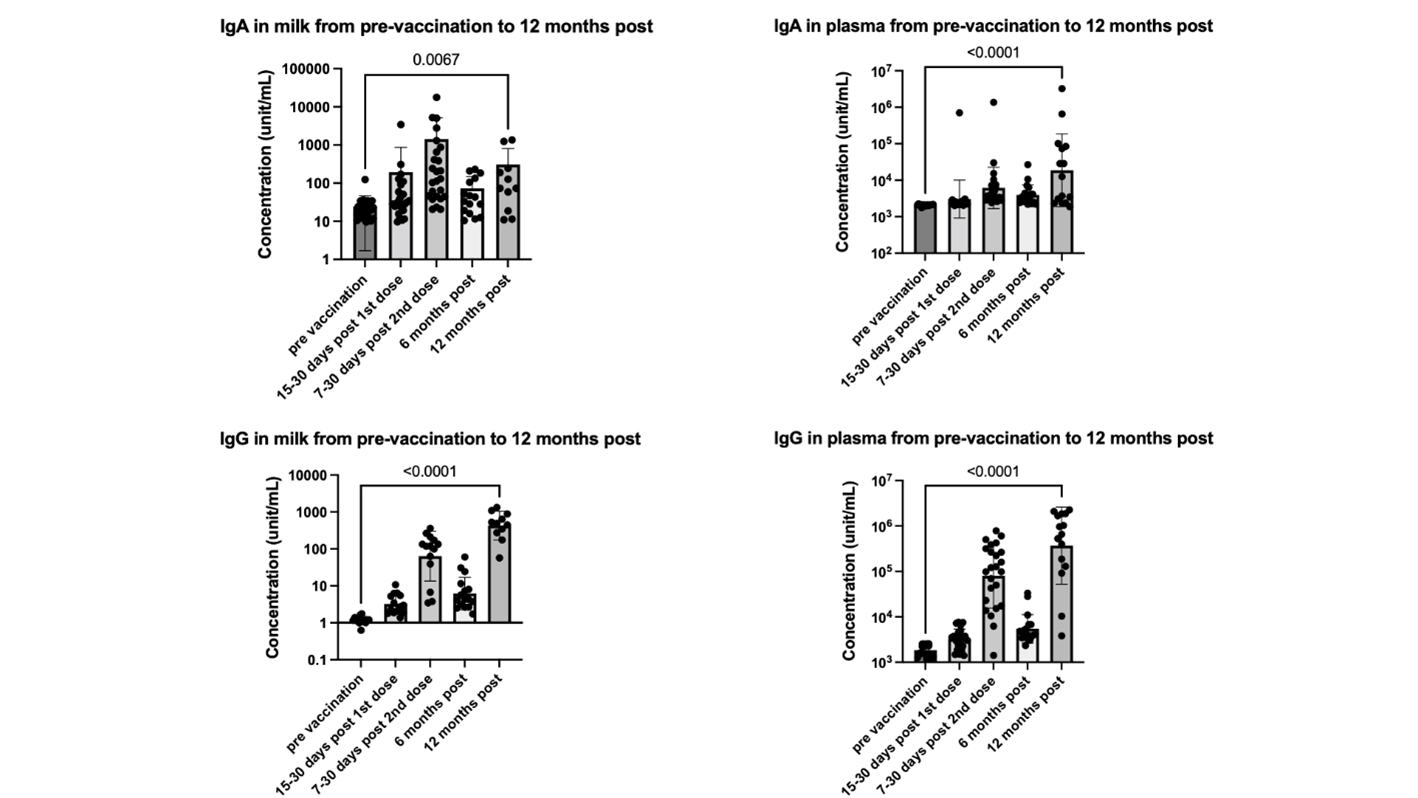
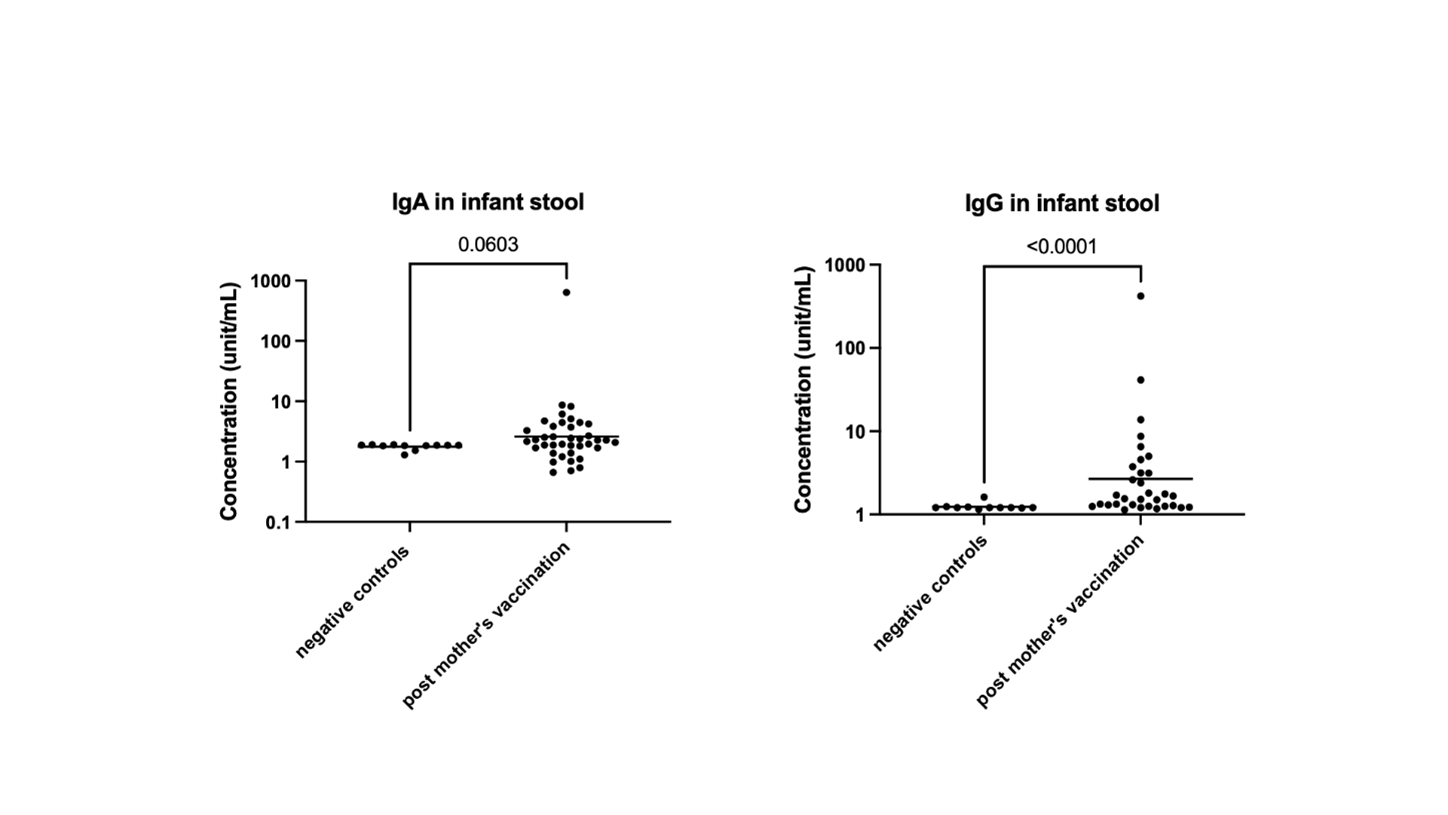
A prospective observational study was conducted at the University of Florida. Thirty-three mothers and 24 infants were enrolled in the original study and followed up to 12 months after the initial vaccination. Of these, 14 mothers received the booster vaccine 8 to 12 months after the initial COVID-19 vaccination. Nine participants received Moderna, and 5 received Pfizer. Maternal milk, plasma, and breastfeeding infants' stools were collected after the booster vaccine, 12 months after the first vaccination. SARS-CoV-2 IgG significantly increases after the mRNA booster, with a dominant response in human milk, plasma, and infants' stool (Fig 1). Post-booster IgG level is significantly higher than pre-vaccination values and 7-30 days post-first mRNA vaccination (Fig 2). Although there is no statistically significant increase in infants' stool IgG post-booster, likely due to a decrease in milk intake by 12 months, there is a significant difference when comparing negative controls to all post-maternal vaccination samples (Fig 3). Using Spearman correlations, we also find that SARS-CoV-2 antibodies were positively correlated in milk and plasma (p=0.03 for IgA and p=0.004 for IgG).
Results:
Conclusion(s):
Among the human milk immune factors, the most abundant ones include sIgA, followed by IgG. Our study found a unique phenomenon: higher SARS-CoV-2 IgG response to mRNA vaccination in human milk, which is reflected in more elevated SARS-CoV-2 IgG antibodies in breastfeeding infants' stool. Although the actual physiologic effects of these antibodies in infants have not yet been elucidated, their presence could be relevant for infant COVID-19 protection. Therefore, well-powered studies are required to understand the value of milk IgG in infant and mammary health.