Health Services Research
HSR 2: Clinical Explorations, Associations, and Interventions
585 - Bronchodilator and Corticosteroid Use Following First Lower Respiratory Tract Infection in Young Children
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 585
Publication Number: 585.223
Publication Number: 585.223
Joy Huang, University of Colorado School of Medicine, Aurora, CO, United States; Angela Moss, University of Colorado, Aurora, CO, United States; Brandy M. Hoyt, Children's Hospital Colorado, Aurora, CO, United States; John Watson, University of Colorado School of Medicine, Denver, CO, United States; Mark Brittan, University of Colorado School of Medicine, Aurora, CO, United States

Joy Huang, BS (she/her/hers)
Medical Student
University of Colorado School of Medicine
Aurora, Colorado, United States
Presenting Author(s)
Background: Despite recommendations against use of inhaled bronchodilators (IB) and oral corticosteroids (OCS) for young children with lower respiratory tract infections (LRTIs), providers continue to overuse these therapies. We aimed to assess prevalence of these treatments and to identify factors associated with their clinical use.
Objective: To identify factors associated with IB and OCS use in children < 2 years old presenting with first LRTI.
Design/Methods: Retrospective cohort study using 2009 – 2019 Colorado All Payer Claims Data of children < 2 years old with at least one LRTI. LRTIs included all claims for bronchiolitis, pneumonia, and wheezing in any clinical setting. Children with prior or concurrent asthma diagnosis were excluded. The primary outcomes were either an IB or OCS prescription claim within 30 days of first LRTI. The primary predictors were IB claim (for IB outcome) or OCS claim (for OCS outcome) prior to the first LRTI. Covariates included age, gender, insurance, family history (FH) of asthma, personal history of atopy, prior use of inhaled corticosteroids (ICS), or complex chronic condition (CCC). Separate multivariable logistic regression models were used to examine the association between previous claims and repeat claims within 30 days of index LRTI for each outcome.
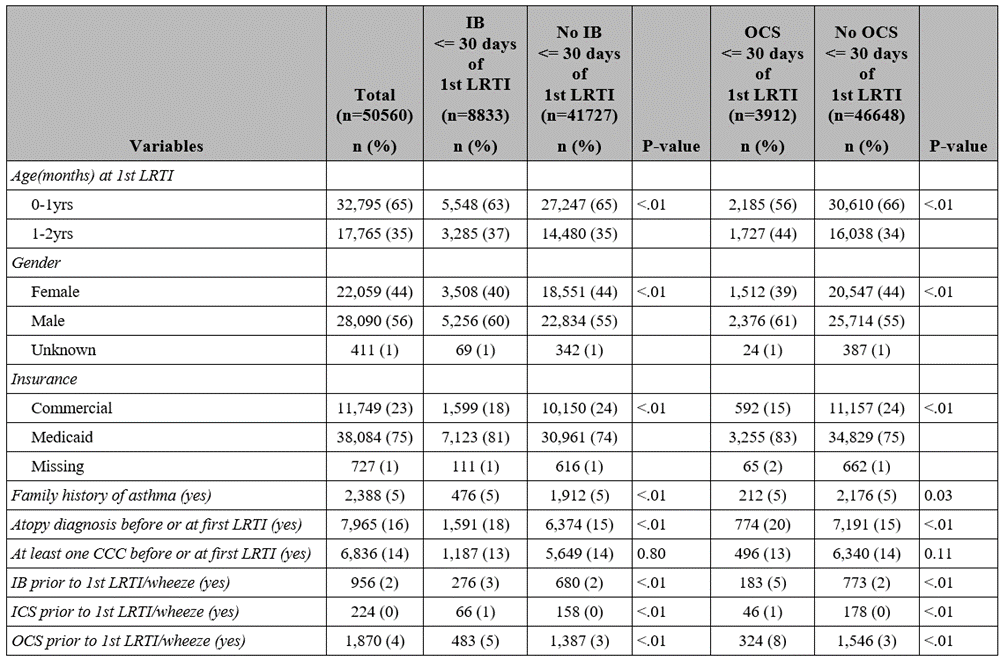
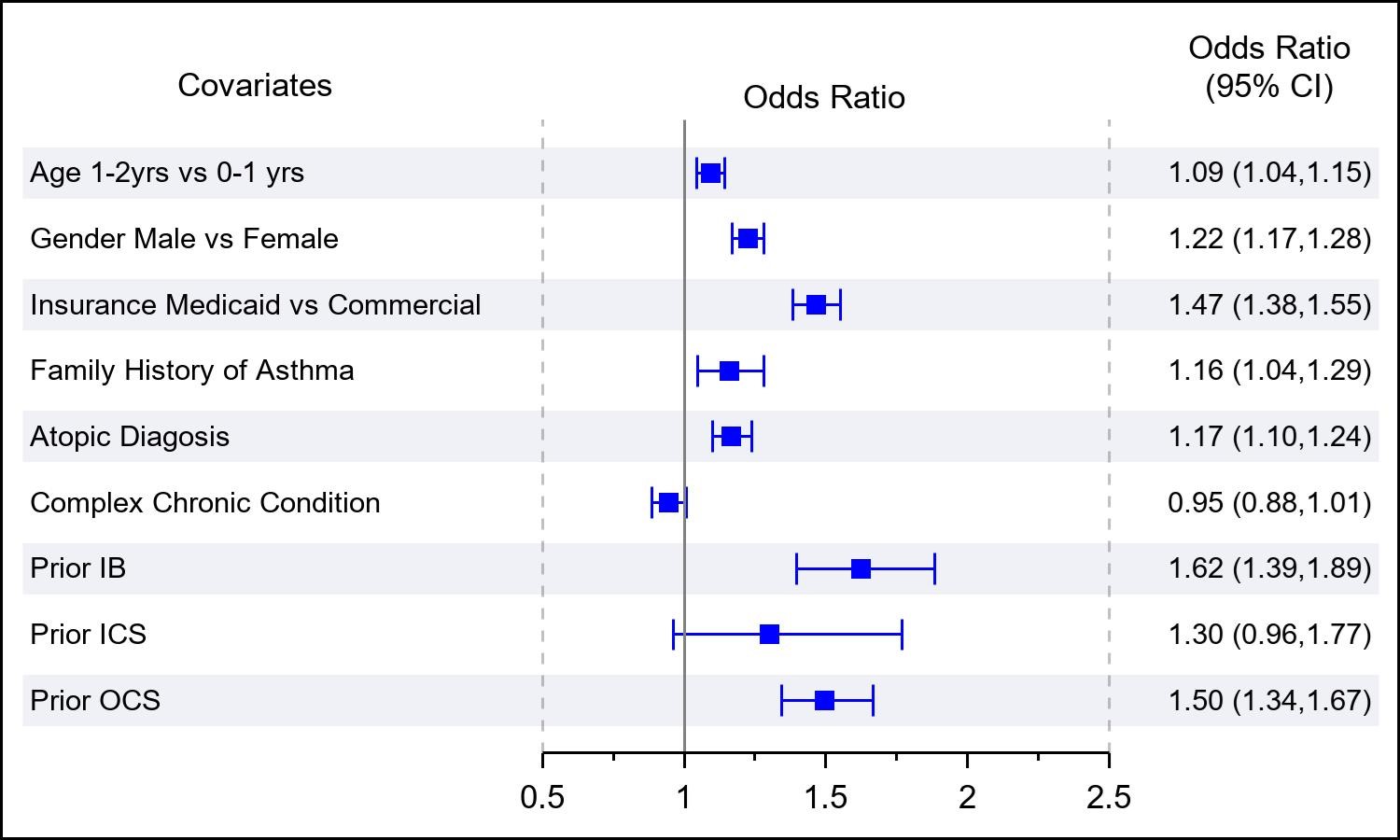
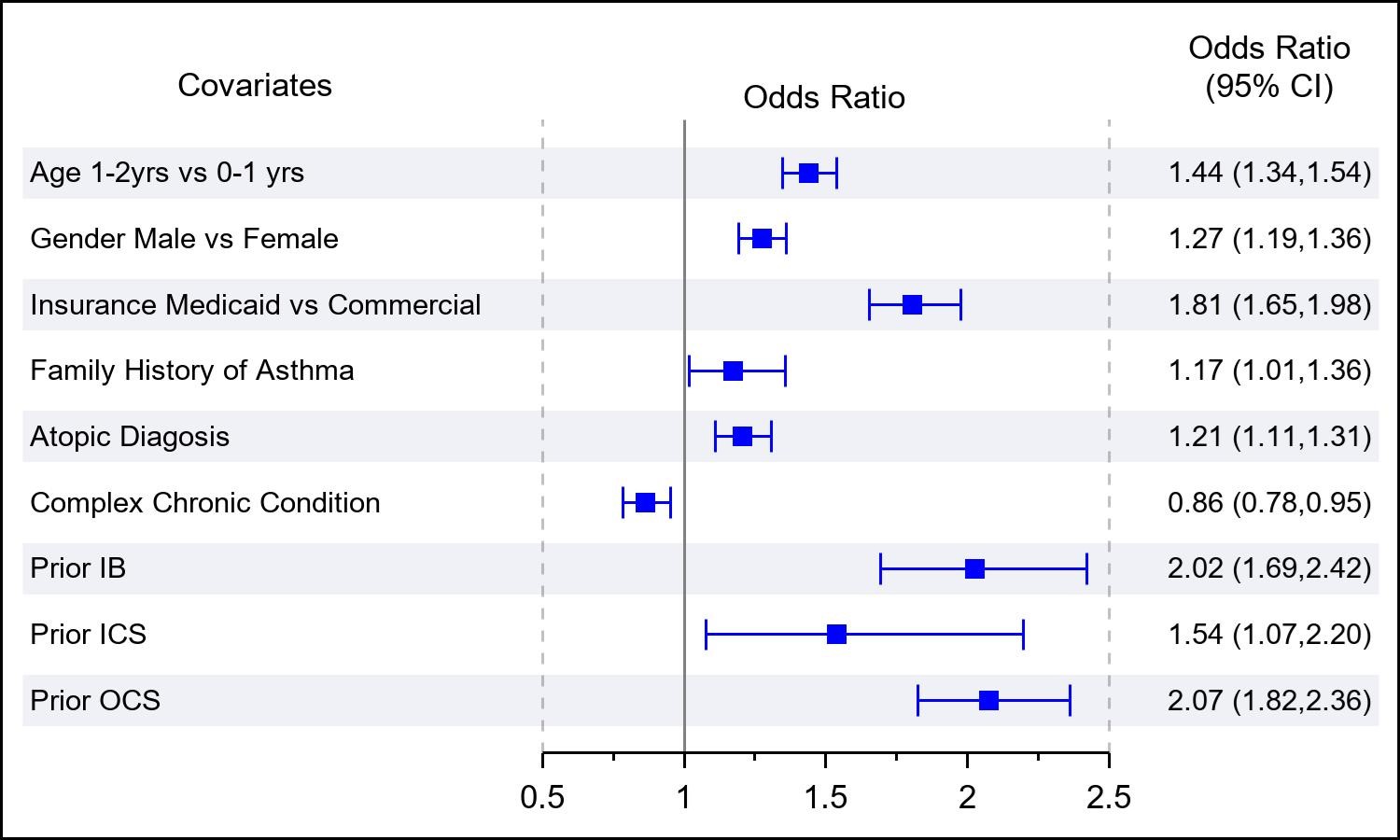
Results: Of 50,560 children with at least 1 LRTI diagnosis, 8,833 (17%) had an IB claim and 3912 (8%) had an OCS claim within 30 days of their first LRTI. Bivariate comparisons between those with and without an IB or OCS claims are shown in Table 1. In adjusted analysis, there was a significant association between previous IB use and IB use within 30 days of first LRTI (odds ratio [OR]: 1.6; 95% CI, 1.4-1.9). Other significant associations with IB use included male gender, Medicaid insurance, asthma FH, atopy, and prior OCS use (Figure 1). There was also a significant association between previous OCS use and OCS use within 30 days of first LRTI (odds ratio [OR]: 2.1; 95% CI, 1.8-2.4). Other significant associations with OCS use included male gender, Medicaid insurance, asthma FH, atopy, and prior IB use (Figure 2).
Conclusion(s): The prevalence of prescribing both IB and OCS at first LRTI is high relative to current recommendations. Prior use of either IB and OCS is associated with reuse of these medications after first LRTI. Quality improvement work would be aided by an understanding of this prescribing momentum in formulating effective de-implementation of these therapies in young children with LRTIs.