Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 1
658 - The Role of Interleukin-1α (IL-1α) in Innate and Adaptive Immune Responses in Recessive Dystrophic Epidermolysis Bullosa (RDEB)
Publication Number: 658.24

Morgan Anderson-Crannage
PhD Candidate
New York Medical College
Montvale, New Jersey, United States- YL
Yanling Liao, PhD (she/her/hers)
New York Medical College
Elmsford, New York, United States
Presenting Author(s)
Co-Author(s)
Background:
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare and devastating disease secondary to mutations in the col7a1 gene that encodes type VII collagen (C7). Infants affected by RDEB are typically born with widespread blistering and areas of missing skin. The repeated cycles of skin erosions and delayed wound healing are accompanied by uncontrollable infections and inflammation, resulting in progressive and mutilating fibrosis such as fusion of the skin between figures and toes. A lethal complication of RDEB is the development of aggressive forms of cutaneous squamous cell carcinoma. How the inflammation is coordinated with fibrotic development to create an immune suppressive dermal microenvironment for cSCC development remains to be elucidated.
Objective: To identify the genes and signaling pathways that are significantly dysregulated in the dermal microenvironment (DME) of RDEB skin that may contribute to the pathological development of RDEB.
Design/Methods:
The paw skin from 11 days old WT and RDEB mice (n=2 per group) was dissociated into single cells and subject to 10x Genomics single cell RNA sequencing (scRNAseq). Cytokines from plasma or skin lysate of RDEB mice were quantitated using ELISA analysis. The human dermal fibroblasts were analyzed for gene expression through flow cytometry analysis.
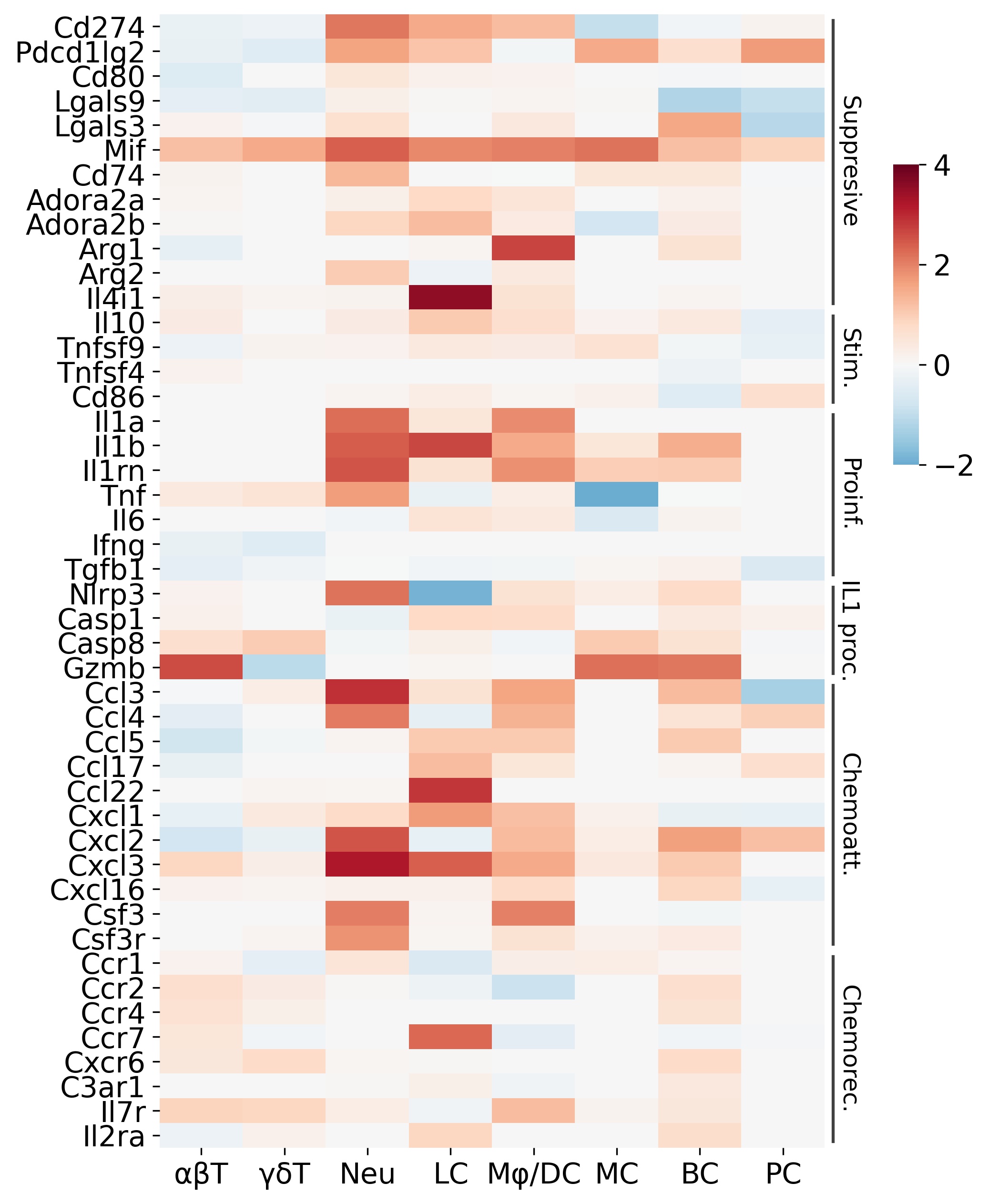
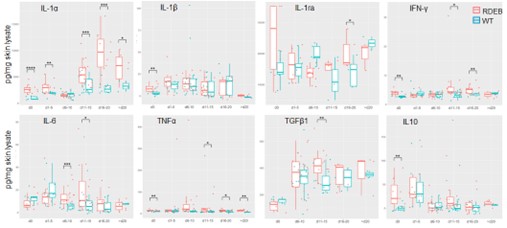
Results: The scRNAseq results revealed a dysregulated RDEB DME with similarities to the features of pro-tumor inflammation, i.e., upregulation of hypoxia signaling and glycolysis enzymes, enhanced angiogenesis and lymphoangiogenesis, and elevation of pro-inflammatory cytokines that are potent mediators of pro-tumor inflammation, such as IL-1α (Fig. 1). The expression of IL-1α was significantly upregulated in multiple immune cell types including neutrophils, macrophages, and Langerhans cells. Importantly, we also identified a specific subset of RDEB fibroblasts that recapitulates the inflammatory cancer associated fibroblasts (iCAFs), based on its upregulation of specific IL-1 signaling induced genes. Supportively, IL-1α was the most significantly and persistently elevated cytokine in the RDEB skin lysate from birth to adulthood (p < 0.01), as compared to other cytokines such as IL-6 or TNF (Fig 2).
Conclusion(s):
Our results suggest that preceding malignant transformation, the RDEB DME has been shaped toward a tumor-permissive state resembling TME. IL-1α is likely the master regulator that drives the inflammatory responses in RDEB. Future studies will determine the effects of IL-1 blockade on the disease progression of RDEB.