Hematology/Oncology
Hematology/Oncology 2
159 - Perception of Chemotherapy-Induced Nausea and Vomiting Control in Pediatric Patients
Publication Number: 159.22

Melissa Rubin, MD (she/her/hers)
Resident
NewYork-Presbyterian Morgan Stanley Children's Hospital
New York, New York, United States
Presenting Author(s)
Background:
Chemotherapy-induced nausea and vomiting (CINV) significantly impacts quality of life. Clinical practice guidelines developed by the Pediatric Oncology Group of Ontario recommend a combination of dexamethasone, 5HT3 antagonists, and NK1 antagonists, dependent on chemotherapy emetogenicity. While guidelines exist, they are not utilized for all patients. Few studies have examined pediatric patient perception of CINV control.
Objective:
Explore patient/family perceptions of CINV management and understand how perception is associated with patient demographics and guideline concordance.
Design/Methods:
English and Spanish-speaking pediatric oncology patients admitted for scheduled chemotherapy were recruited during all admissions to complete a survey assessing CINV management. Surveys were completed by the patient or caregiver, if the patient was too young or unable to participate. Likert scales (0= not well controlled; 4= extremely well controlled) assessed CINV symptom management during the current admission. Key demographics were used to understand association with perceptions and adherence to anti-emetic guidelines with Chi-Square and analyzed using Stata 14.2.
Results:
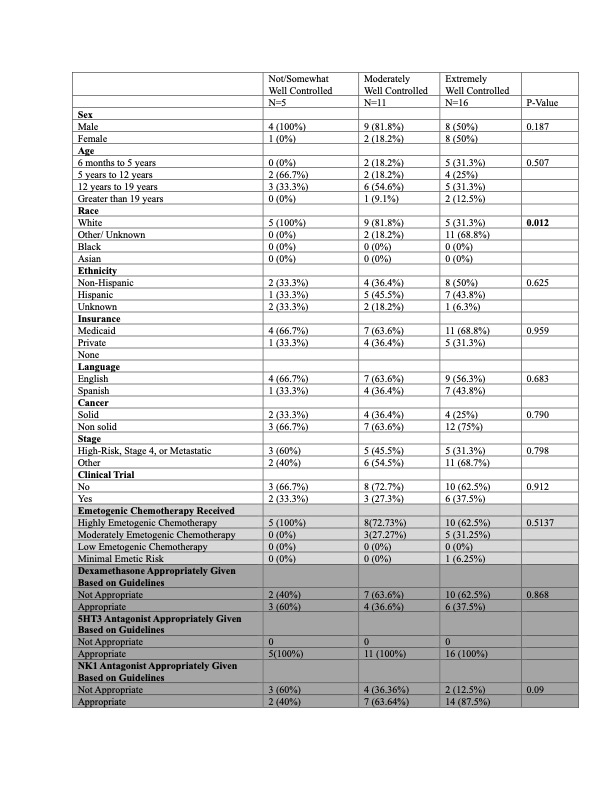
A total of 32 surveys were distributed to 16 unique patients/ families over 10 months. For nausea control: 7 encounters reported not/ somewhat well controlled, 11 moderately well, 14 extremely well (Table 1). For vomiting control: 5 encounters reported not/ somewhat well controlled, 11 moderately well, 16 extremely well (Table 2). For both nausea and vomiting symptoms, there was a difference in perceived CINV control by patient-reported race, however, 41% of patients reported “other/unknown race” of which 1 declined to answer, and 12 described other combinations not described. There were no significant differences in patient-reported CINV across other demographics. There were no significant differences in patient-reported CINV when considering emetogenicity of chemotherapy received, or concordance to guidelines, which varied between receiving concordant care 40% of the time for dexamethasone, 71% for NK1 antagonists, and 100% for 5HT3 antagonists.
Conclusion(s):
The majority of responses reported CINV symptoms were moderately- or extremely- well controlled. While CINV prophylaxis did not always follow guidelines, guideline concordant care was not associated with perception of CINV control. Future studies will expand upon this novel patient/ family perception to include a larger sample size to better understand differences by demographic and clinical factors, including the impact of guideline concordant anti-emetic prophylaxis. .jpg)