Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 1
656 - ScRNA-seq reveals novel functional subsets and TNF responsiveness of chorio-decidua macrophages during intrauterine infection in the Rhesus macaque
Publication Number: 656.24

Neema Pithia, MD (she/her/hers)
Fellow
UCLA Mattel Childrens Hospital
Los Angeles, California, United States
Presenting Author(s)
Background:
Intrauterine infection (IUI) triggers inflammation at the maternal-fetal interface that leads to preterm birth. Skewing of macrophage polarization towards pro- or anti-inflammatory function (M1/M2) is likely important in balancing host-defense with preventing fetal-allograft rejection. We have reported that Adalimumab (Adal) (anti-TNF Ab) decreased inflammation in IUI.
Objective: We hypothesized that chorio-decidua macrophage polarization will change towards a pro-inflammatory phenotype in a TNF-signaling dependent manner during IUI.
Design/Methods: IUI was induced by intraamniotic lipopolysaccharide (IA LPS). Rhesus macaque groups at ~80% gestation were: 1) Controls (IA saline) 2) IA LPS 3) IA LPS+Adal (IA+subcutaneous) with delivery 16h after exposures. Tissue harvest and chorio-decidua (CD) cell suspension was prepared after digestion. Bulk RNA-seq (n=6/group) was performed on FACS-sorted (Live/CD45+CD3-CD14highHLA-DR+) CD macrophages and single-cell (sc) RNA-seq (control n=2, LPS n=3, Adal+LPS n=3) on CD cells using 10X genomics and Bioinformatics analyses.
Results:
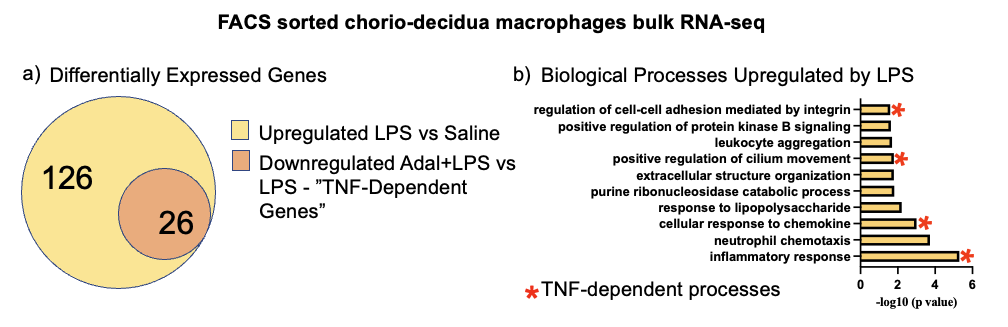
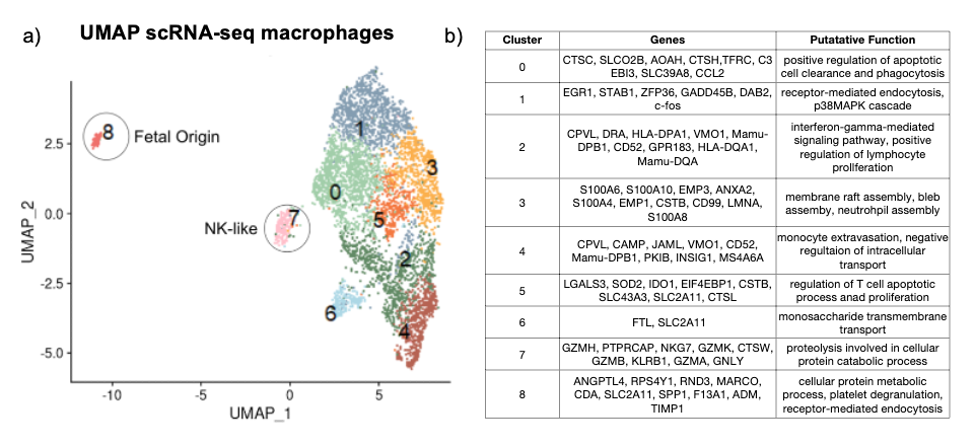
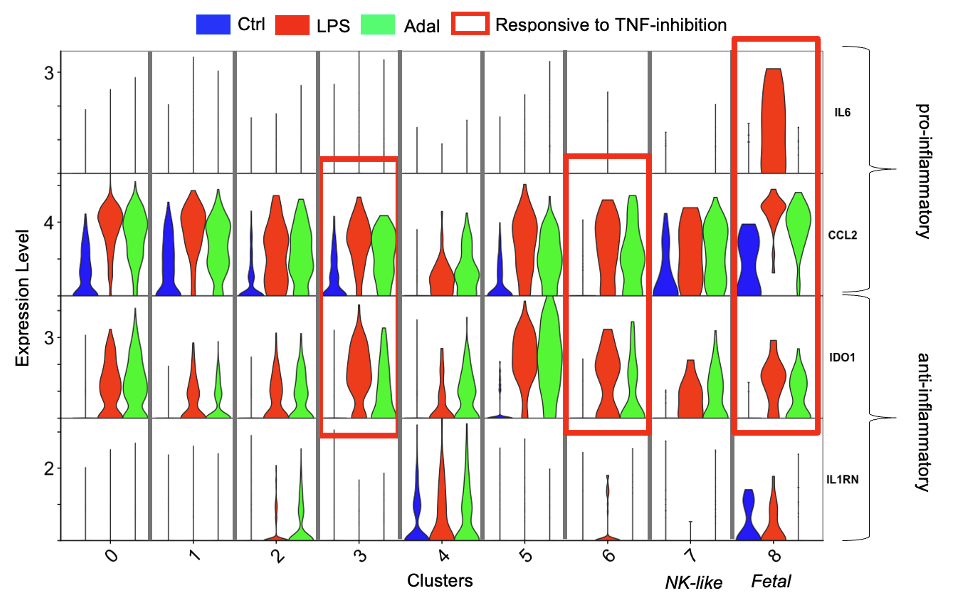
Bulk RNA-seq showed 126 genes upregulated by LPS vs. Control in FACS sorted CD macrophages, of which 26 (21%) were downregulated by Adal (TNF-dependent genes) (Fig 1a). ~40% of the top 10 biological processes were downregulated by Adal (Fig 1b). ScRNA-seq revealed 9 clusters (Fig 2a). Only cluster 8 expressed the Y-chromosome gene RPS4Y1 (Fig 2b). Cluster 7 expressed intracellular granule genes Granzyme B and Granulysin (Fig 2b). LPS-exposure increased gene expression of pro-inflammatory CCL2 (Monocyte Chemotactic Protein 1) and anti-inflammatory IDO1 (Indoleamine 2,3-Dioxygenase) (Fig 3). Pro-inflammatory IL6 (Interleukin 6) gene expression was exclusively induced by LPS in cluster 8 (Fig 3). Adal decreased LPS-induced CCL2 and IDO1 expression selectively in clusters 3, 6, and 8 (Fig 3), but did not change expression of the LPS-induced anti-inflammatory gene IL1RN (Interleukin 1 Receptor Antagonist) in clusters 2, 4, and 6 (Fig 3).
Conclusion(s):
Unlike the M1/M2 paradigm, LPS upregulated pro- and anti-inflammatory gene expression in all CD macrophage subsets, suggesting less polarization. Contrary to a presumption of immaturity of fetal immunity, fetal macrophages (cluster 8) had the highest LPS-induced expression of IL6 and CCL2. We demonstrate a novel macrophage subset (cluster 7) expressing NK cell markers. We previously reported that during IUI, CD neutrophil activation is largely TNF-dependent. This study shows that CD macrophage activation is only partially TNF-dependent, informing us of the mechanisms of IUI.