Pediatric Nutrition
Pediatric Nutrition
43 - Concentrations of iron and DHA in infant formula purchased in the US compared to international nutrition requirements – a contradiction exacerbated by the infant formula shortage
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 43
Publication Number: 43.253
Publication Number: 43.253
Alexander Strzalkowski, Boston Children's Hospital, Auburndale, MA, United States; Grace Black, University of Rochester, Rochester, NY, United States; Bridget E. Young, University of Rochester, Rochester, NY, United States
.jpg)
Bridget E. Young, PhD (she/her/hers)
Assistant Professor
University of Rochester
University of Rochester School of Medicine and Dentistry
Rochester, New York, United States
Presenting Author(s)
Background: Prior to the infant formula shortage, European formulas obtained from third-party vendors remained popular despite warnings that these formulas were not approved by the US Food and Drug Administration (FDA). In response to the infant formula shortage of 2022, the FDA granted temporary approval, with options for permanency, to several different foreign infant formulas. These products, which were originally manufactured under different international regulatory standards, are now prominent in the US marketplace. Two micronutrients that differ in requirement regulations between the US and the European Union (EU) are iron and docosahexaenoic acid (DHA).
Objective: To determine the average iron and DHA content of powdered infant formula purchased in the US and compare these concentrations to both US and EU nutrient requirements.
Design/Methods: We acquired national data regarding powdered term infant formula purchased from all major physical store locations between 2017 through 2019 from Information Resources, Inc. Iron and DHA concentrations and scoop sizes for each formula were obtained from manufacturers. Equivalent ounces of prepared formula and weighted average iron and DHA concentrations were calculated.
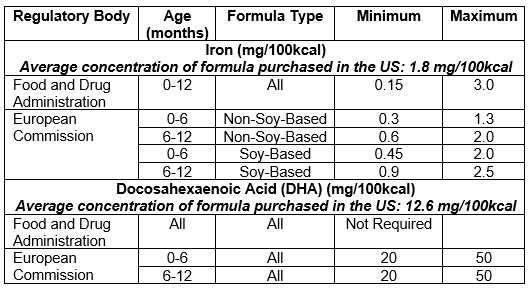
Results: These data represent $8.1 billion of sales over this three-year period, equating to 55.8 billion ounces of formula purchased. US and EU regulations for infant formula iron and DHA concentrations are given in Table 1. The average iron content of all formula purchased was 1.80 mg/100kcal (range = 1.0-1.9 mg/100kcal), which far exceeds the EU maximum allowable concentration for Stage 1 formula (1.3 mg/100kcal). 96% of the formula purchased in the US during the study time period contained an iron content that surpassed the EU Stage 1 maximum for iron. The average DHA content of all formula purchased was 12.6 mg/100kcal (range = 0-17 mg/100kcal). Over 99.9% of formula purchased in the US contained DHA, but no formulas met the minimum DHA requirements set by the EU (20 mg/100kcal).
Conclusion(s): With the influx of foreign formulas into the US marketplace, it is important to note that differences in nutrient requirements have resulted in large differences in nutritional content, particularly for iron and DHA. Medical practitioners should be aware of these differences. These data suggest the timing is right to revisit FDA nutrient requirements for infant formula in the face of a global infant formula economy.