Infectious Diseases
Infectious Diseases 1
624 - SARS-CoV-2 percent positivity and mRNA COVID-19 vaccination coverage among symptomatic children ages 3-17 years— Increasing Community Access to Testing (ICATT) Program, August-December 2022
Publication Number: 624.225
- KF
Katherine E. Fleming-Dutra, MD (she/her/hers)
Physician, Vaccine Effectiveness and Policy Team, Coronaviruses and Other Respiratory Viruses Division
Centers for Disease Control and Prevention
Atlanta, Georgia, United States
Presenting Author(s)
Background:
COVID-19 vaccination is the most important measure to protect children from COVID-19; SARS-CoV-2 transmission contributed to surges in respiratory illnesses among children during the fall of 2022.
Objective:
We analyzed data from a national, pharmacy-based SARS-CoV-2 testing network to examine percent positivity and vaccination coverage among children with symptomatic COVID-19-like illness.
Design/Methods:
We included SARS-CoV-2 nucleic acid amplification tests (NAATs) conducted at 9,710 pharmacy SARS-CoV-2 testing sites in the Increasing Community Access to Testing (ICATT) program during August 1-December 9, 2022 among children ages 3-17 years reporting ≥1 symptom of COVID-19-like illness. Age, symptoms, and vaccination status, including COVID-19 vaccine product and number of doses received and month and year of most recent dose, were reported by caregivers. Tests were excluded from analyses for children with 1) a reported immunocompromising condition, 2) a reported positive SARS-CoV-2 test in the previous 90 days, 3) most recent vaccination date prior to authorization, 4) missing key data elements, or 5) most recent vaccine dose < 2 weeks before the test date. Coverage for mRNA COVID-19 vaccines was estimated among SARS-CoV-2 negative children by age group, categorized as unvaccinated, receipt of ≥1 monovalent vaccine doses but no bivalent dose, and receipt of bivalent vaccine dose. Vaccine doses administered after September 2022 (children 12-17 years) and October 2022 (children 5-11 years) were considered bivalent.
Results:
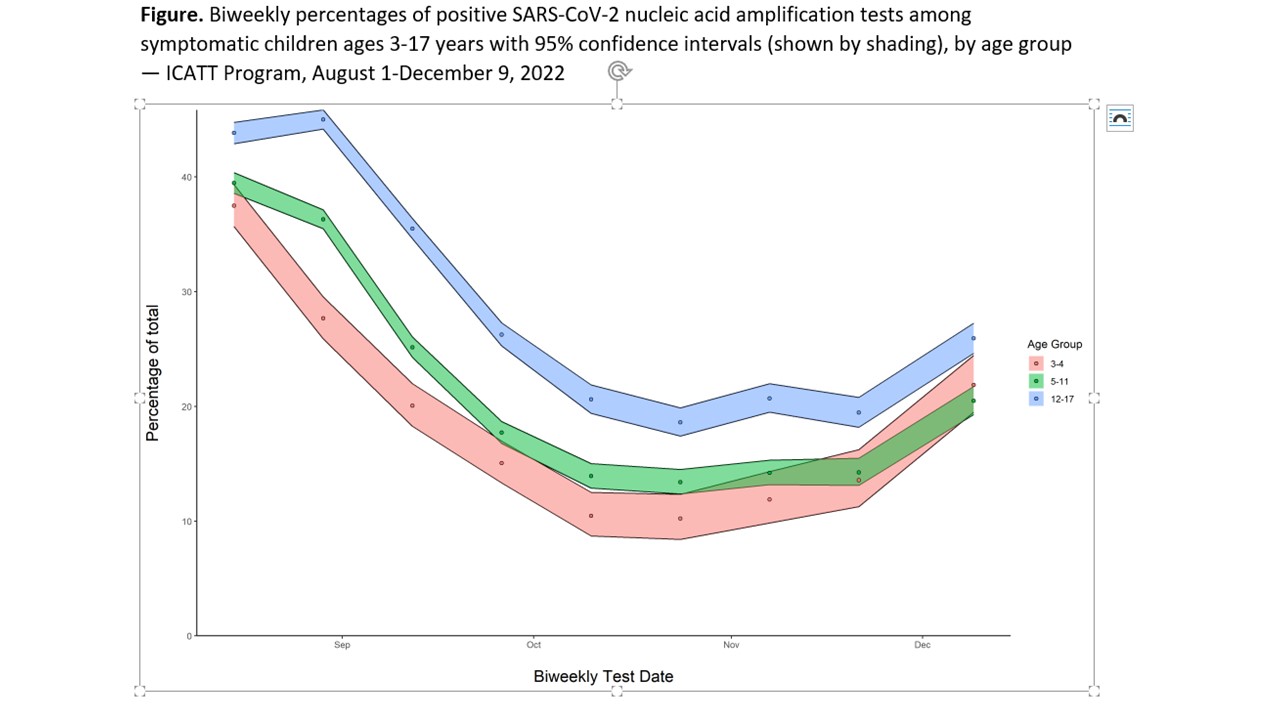
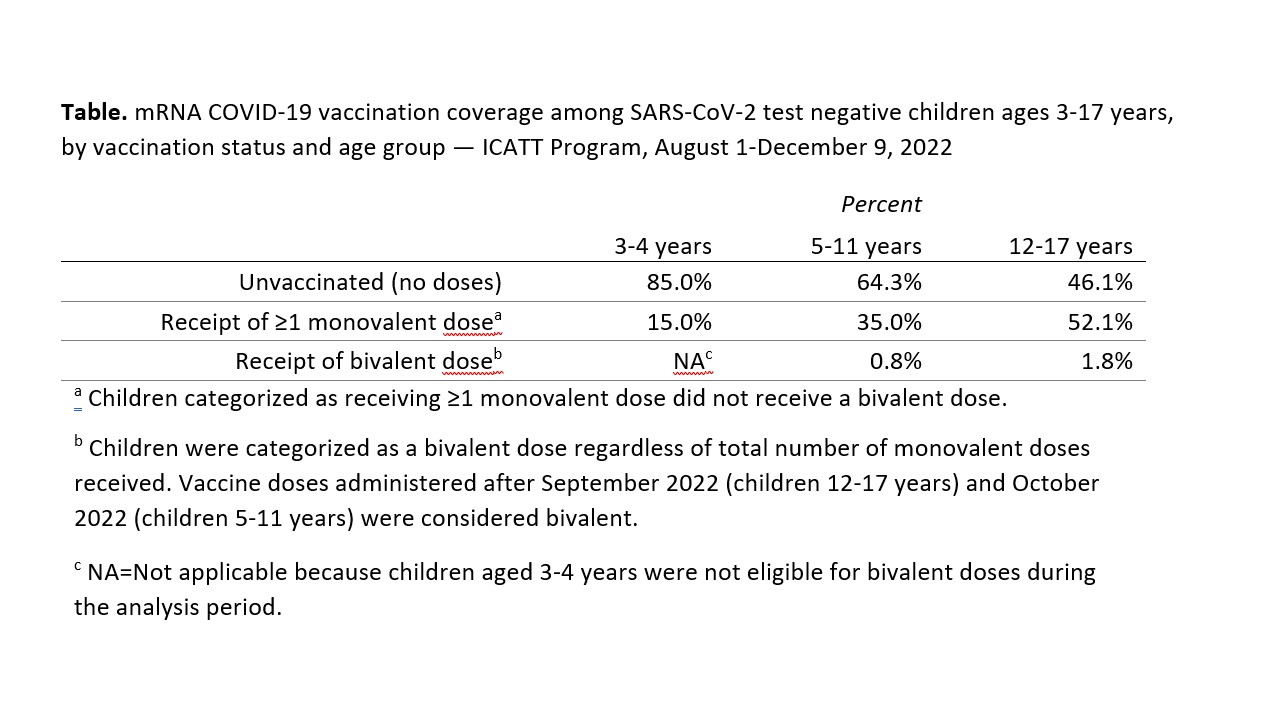
During August 1-December 9, 2022, 13,272 tests were conducted among children ages 3-4 years, 60,855 among children ages 5-11 years, and 64,795 among children ages 12-17 years. SARS-CoV-2 percent positivity in symptomatic children was highest in August, decreased to about 10% by October, and increased in November (Figure). Percent positivity was higher among 12–17-year-old children throughout, but similar among children 3-4 years and 5–11 years by October-December. Among SARS-CoV-2 negative children, vaccine coverage was highest among adolescents ages 12-17 years; bivalent dose coverage was low in all age groups (Table).
Conclusion(s):
During the fall 2022 respiratory illness surge, ≥10% of children ages 3-17 years who presented for SARS-CoV-2 testing with COVID-19-like illness had SARS-CoV-2 infection. Continued access to SARS-CoV-2 testing is needed to inform isolation and public health measures. Opportunities exist to improve COVID-19 vaccine coverage among children, especially for bivalent doses.