Emergency Medicine: All Areas
Emergency Medicine 6
352 - Elevated acetaminophen in suspected suicidal pediatric ingestions
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 352
Publication Number: 352.21
Publication Number: 352.21
Taylor White, Nationwide Children’s Hospital, Cardington, OH, United States; Natalie I. Rine, Nationwide Children's Hospital, Columbus, OH, United States; Marcel J. Casavant, Nationwide Children's Hospital, columbus, OH, United States; Sara Helwig, Nationwide Children's Hospital, Columbus, OH, United States; Matthew B. Wysong, Nationwide Children's Hospital, Dublin, OH, United States; Jess Flowers, Nationwide Children's Hospital, Columbus, OH, United States; Leah K. Middelberg, Nationwide Children's Hospital, Columbus, OH, United States

Taylor M. White, DO (she/her/hers)
Pediatric Emergency Medicine Fellow
Nationwide Children’s Hospital
Columbus, Ohio, United States
Presenting Author(s)
Background: Early recognition and treatment of acetaminophen (APAP) overdose is crucial as it continues to be the leading cause of acute liver failure in the United States. Adult studies conflict in supporting universal APAP screening for patients who are alert and oriented upon presentation for intentional ingestion of reportedly non-APAP substances. These studies fail to include the majority of the vulnerable adolescent and preteen population, in whom APAP remains the leading agent ingested in intentional overdoses. This study presents the first pediatric-specific data on the rate of APAP discovery on screening labs of children presenting for intentional ingestion.
Objective: This study aimed to measure the rate of elevated APAP levels in pediatric patients presenting to an emergency department (ED) after an intentional ingestion in which APAP was not initially reported and, of those patients, how many required treatment with N-acetylcysteine (NAC).
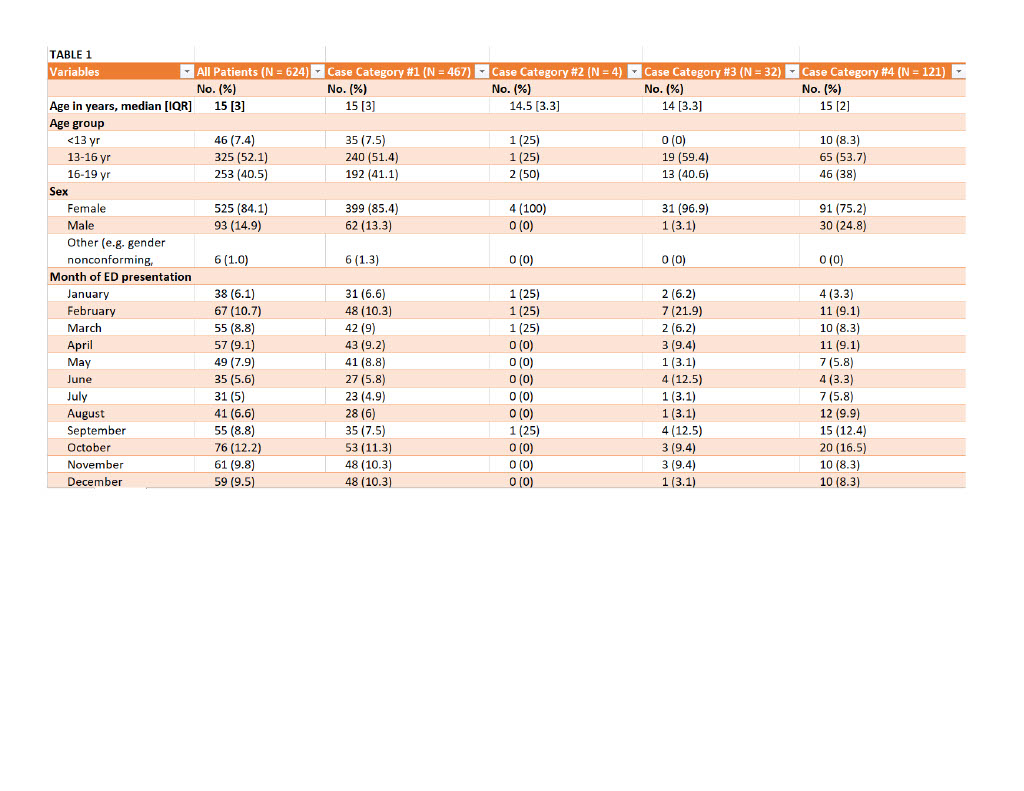
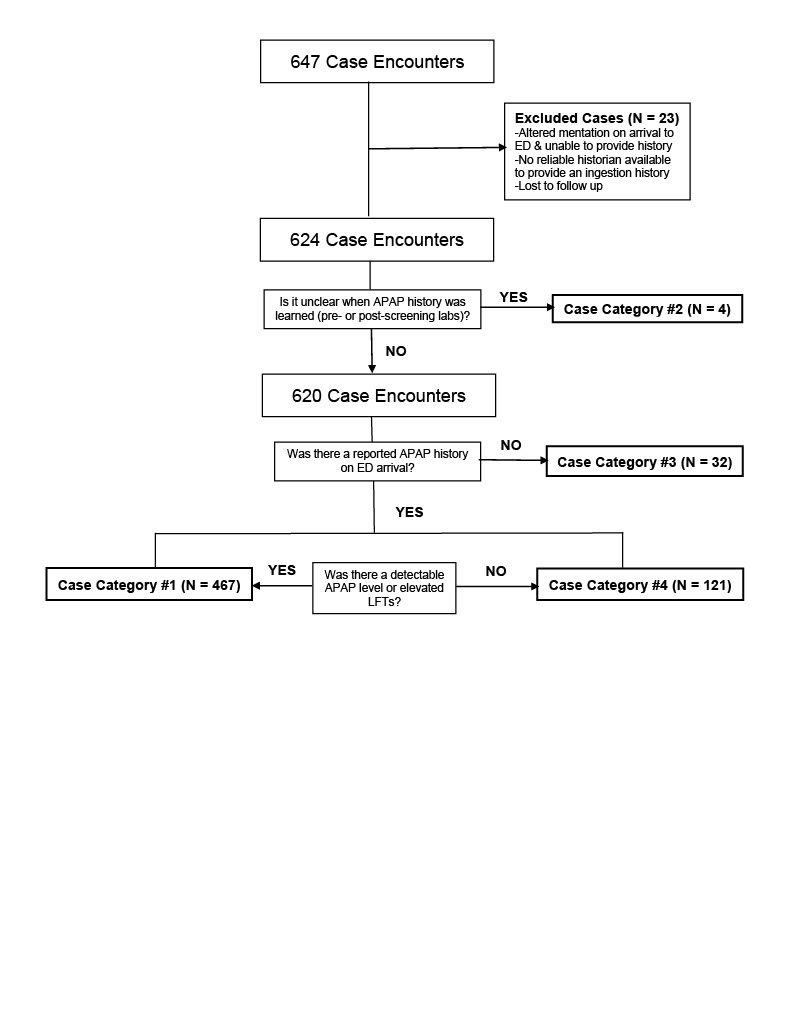
Design/Methods: A retrospective chart review was conducted utilizing the Central Ohio Poison Center database for all patients ≤19 years of age who visited a regional ED for an intentional ingestion between January 2021 and December 2021. Patients were included if they gave an initial history of intentional APAP ingestion, if a measurable APAP level was detected during their visit, or if transaminases were elevated on screening labs indicating suspicion for APAP ingestion. Patients were excluded if they presented to an ED altered and unable to relay a history or did not have a reliable historian to relay an ingestion history. Medical outcomes were recorded, classifying patients into four groups based on reported history and initial ED screening labs.
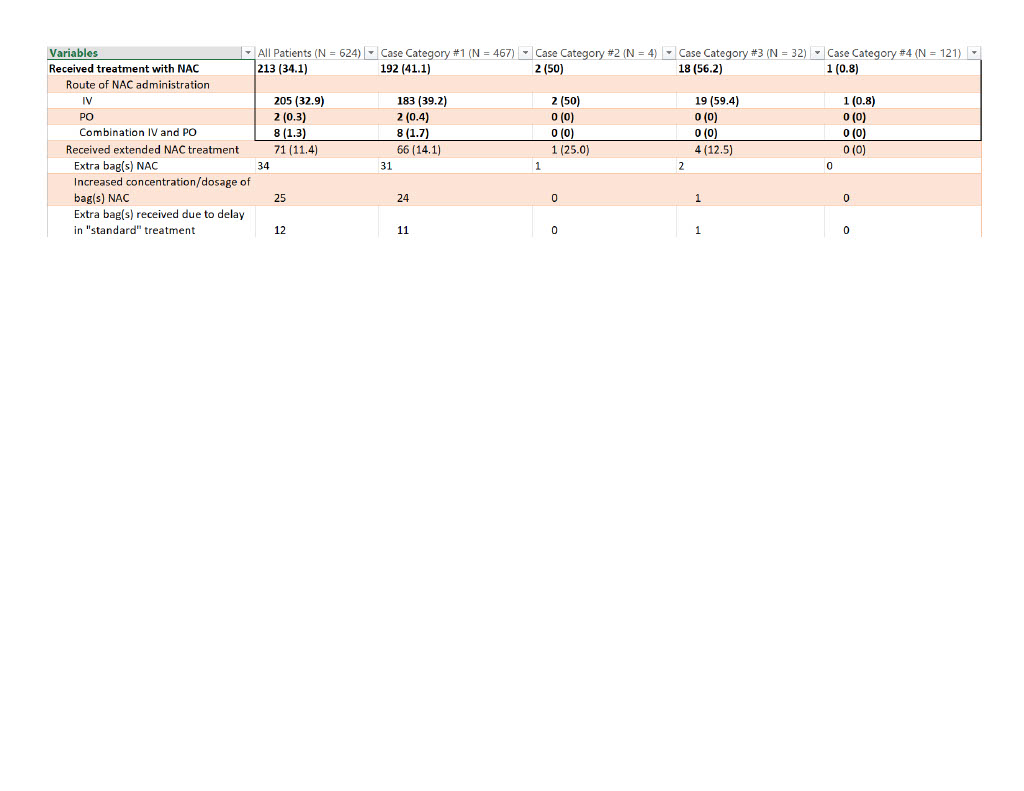
Results: Of the 647 patients, 23 were excluded due to altered mentation, lack of screening lab results, or lost to follow up. Of the remaining 624 patients, 32 (5.1%) had no reported APAP history on ED arrival but had a detectable APAP level or elevated transaminases on initial screening labs. Of those 32 patients, 18 (56.3%) received treatment with NAC and 4 (22.2%) required extended NAC treatment. No patients were referred for liver transplant and no patients died.
Conclusion(s): Although data demonstrated a relatively low frequency of detectable APAP and/or elevated transaminases with an unreported APAP history, the risk of missing APAP overdose is unnecessary given accessible treatment to prevent or limit potentially fatal hepatotoxicity. This study supports universal APAP screening in all pediatric intentional ingestions despite history provided on ED presentation.