Infectious Diseases
Infectious Diseases 1
628 - Use of Monoclonal Antibodies for the Treatment of COVID-19 in Pediatric Patients: Single Center Study
Publication Number: 628.225

Julia Cheng (she/her/hers)
Medical Student
USF Health Morsani College of Medicine
Tampa, Florida, United States
Presenting Author(s)
Background:
The COVID-19 pandemic demanded the timely development of new therapeutics for prevention and treatment of COVID-19 infection. Aside from new vaccines, monoclonal antibodies (mAb) emerged as alternative treatment of COVID-19 in high-risk patients. Initially, there was no evidence for the safety or efficacy of mAb therapy for treatment of COVID-19 in pediatric patients. All mAb therapies available during the pandemic were approved under emergency authorization for use in pediatric patients.
Objective: To describe the safety of mAb use as COVID-19 treatment in pediatric patients, including those with risk factors.
Design/Methods:
Pediatric patients < = 18 years old who received bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab for treatment of mild-to-moderate COVID-19 disease at Tampa General Hospital from 1/1/2021 to 12/31/2021 were identified retrospectively by electronic medical record (EMR). Descriptive statistics were used to describe the population and outcomes.
Results: A total of 141 pediatric patients received mAb therapy during the study period. Patient data including demographics, adverse events, and outcomes were extracted from patients’ EMR. Participants ages ranged between 10 months and 18 years. There were two patients below age 12 years who received mAb under compassionate use. Five (3.6%) received bamlanivimab, 9 (6.4%) received bamlanivimab-etesevimab, and 127 (90.1%) received casirivimab-imdevimab. Most patients were white (n=82, 58.2%). There were also African-American (n=16, 11.4%) and Hispanic (n=16, 11.4%) patients. Common risk factors in this population included asthma (n=38, 26.95%), obesity (n=29, 20.57%), and immunocompromised state (n=8, 5.7%). Two patients (1.4%) experienced infusion-related adverse events, consisting of nausea and vomiting. Within 90 days of receiving mAb, only 3 patients (2.1%) required additional medical care for ongoing COVID-19 symptoms. None of those patients needed to be hospitalized or received escalation of care. There were no variations identified in clinical response according to race/ethnicity.
Conclusion(s):
mAb were well-tolerated therapies for high-risk pediatric patients infected with COVID-19. The most common comorbid conditions associated with receipt of mAb therapy were asthma, obesity, and immunocompromised state. In our study cohort, pediatric patients that received mAb did not experience hospitalization post-infusion.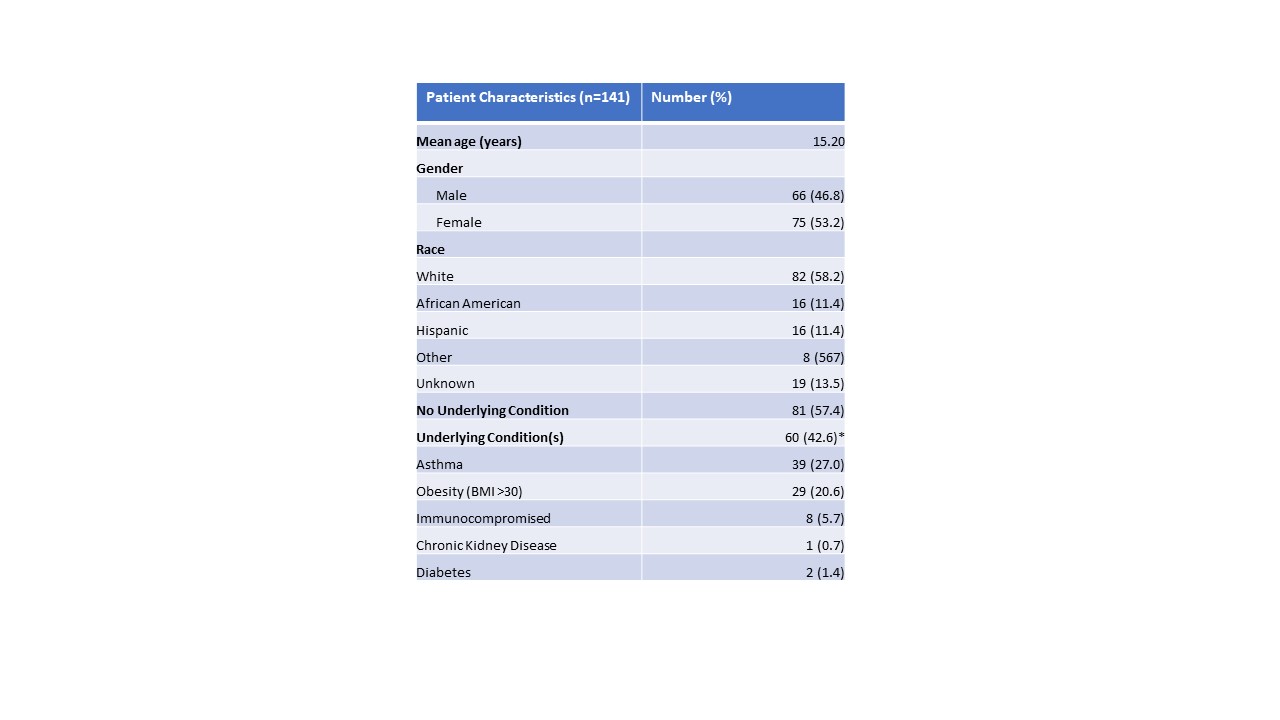
.jpg)
