Neonatal Infectious Diseases/Immunology
Neonatal Infectious Diseases/Immunology 1
648 - CD3/CD28 stimulation promotes regulatory T cell expansion and function in extremely premature infants
Saturday, April 29, 2023
3:30 PM - 6:00 PM ET
Poster Number: 648
Publication Number: 648.24
Publication Number: 648.24
Timothy J. Boly, University of Iowa Stead Family Children's Hospital, Iowa City, IA, United States; Jennifer Bermick, University of Iowa, Iowa City, IA, United States

Timothy J. Boly, DO (he/him/his)
Fellow Physician
University of Iowa Stead Family Children's Hospital
Iowa City, Iowa, United States
Presenting Author(s)
Background: Extremely premature infants have altered immune responses, which makes them more susceptible to infections. The role of the adaptive immune system in these responses has been incompletely characterized.
Objective: Determine differences in T cell expansion and function between extremely premature infants, late preterm infants, term infants, and adults.
Design/Methods: Residual blood samples from extremely preterm infants (22-26 weeks’ gestation) and cord blood samples from late preterm ( >33 weeks’ gestation) and term infants ( >37 weeks’ gestation) were collected. Adult blood samples were collected from healthy volunteers. CD4+ T cells were isolated and stimulated with anti-CD3/anti-CD28 beads with 50 ng/mL IL-2 for 72 hours. Following stimulation, flow cytometry was used to evaluate surface CD3, CD4, CD8, and CD25 expression. qPCR was performed to evaluate transcriptional changes at 24 and 72 hours for genes related to Th1, Th2, Th17, and Treg function. A multiplex protein assay was used to assess T cell production of IFN-γ, IL-2, sIL2Rα, IL-4, IL-5, IL-9, IL-10, IL-13, IL-17, TNF-α, and TGF-β1 in the culture medium at 24 and 72 hours.
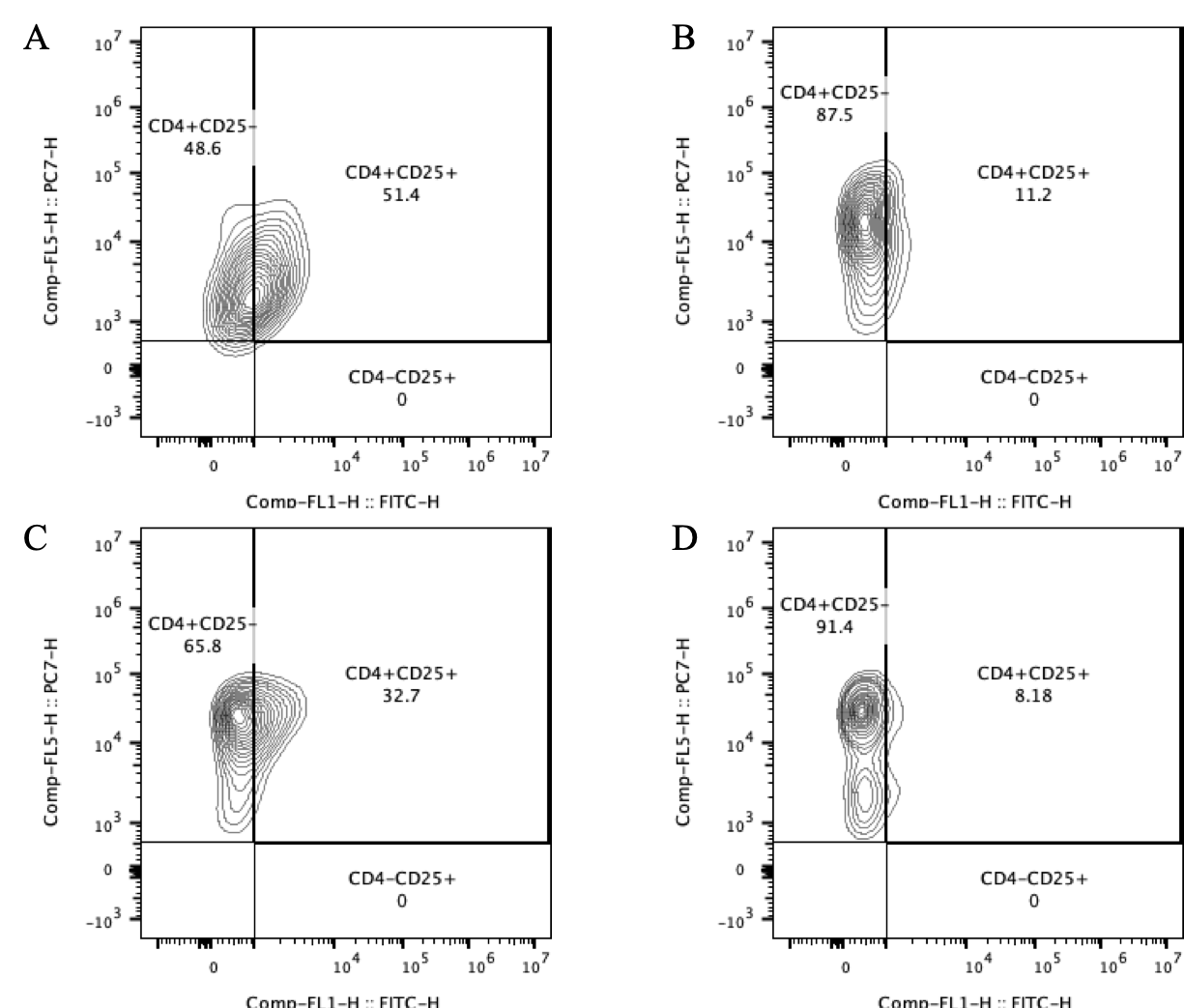
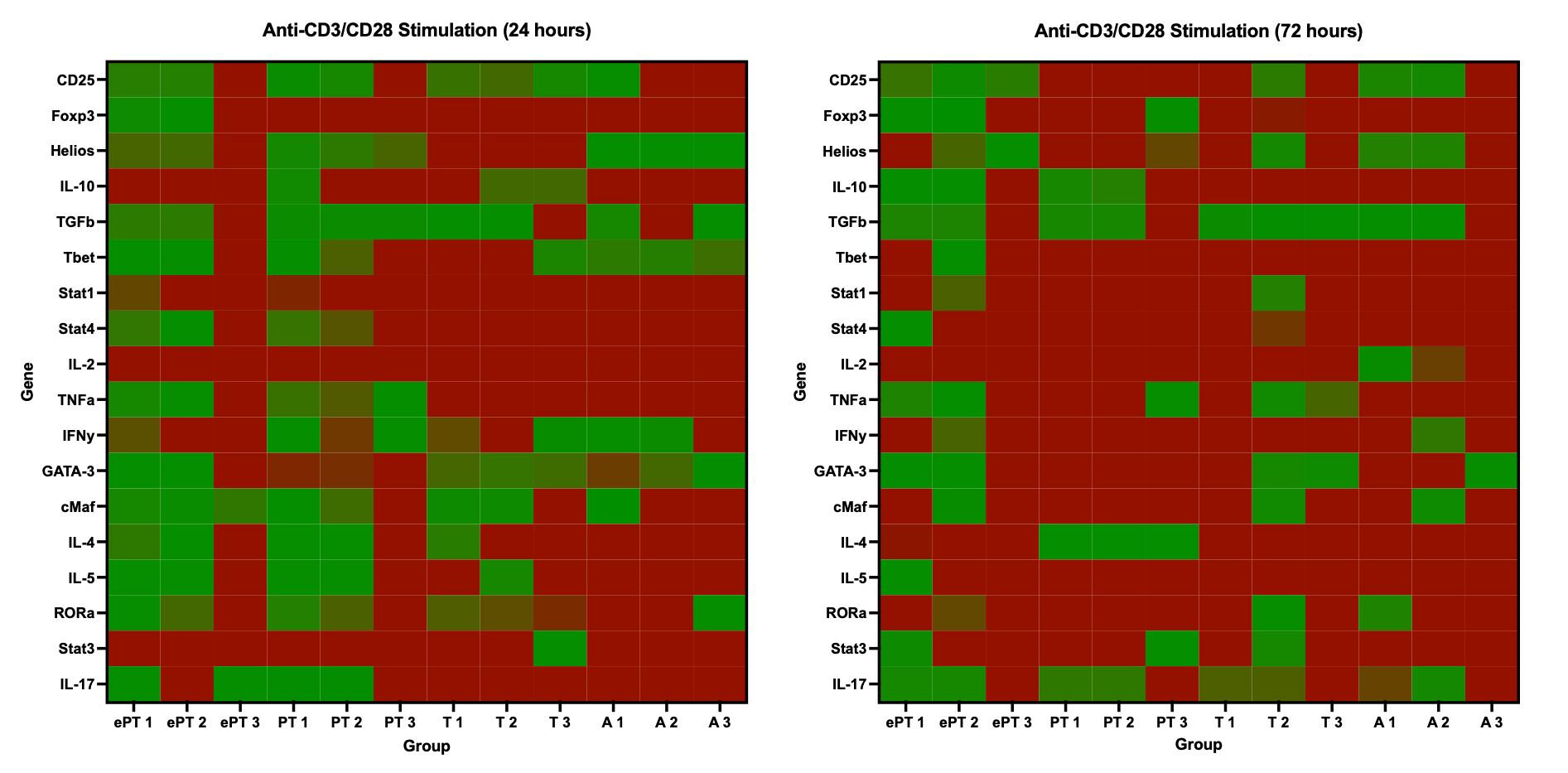
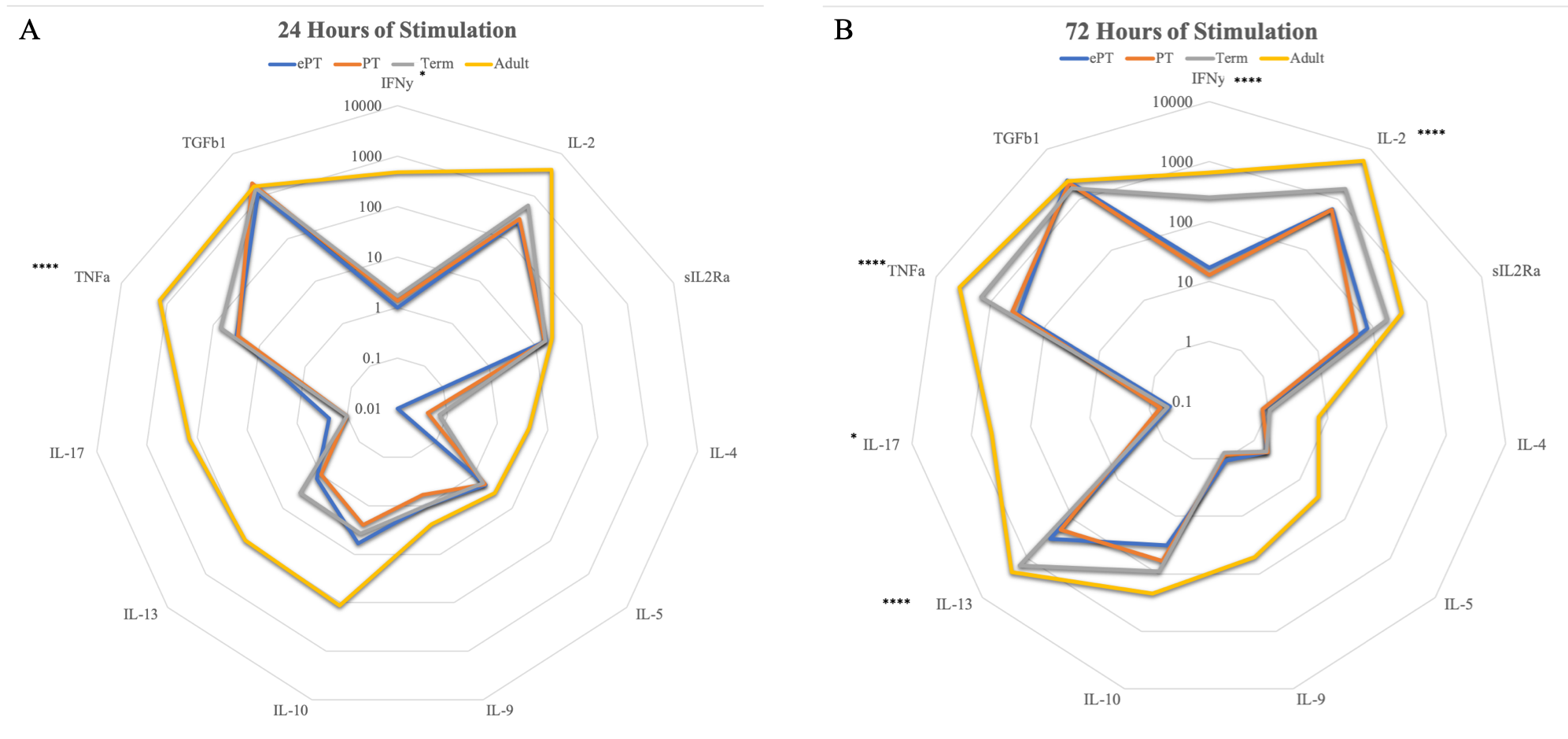
Results: Extremely preterm T cells had significantly higher proportions of Tregs following stimulation as compared to the other age groups(Figure 1). Following stimulation at 24 and 72 hours, extremely premature T cells were less likely to upregulate pro-inflammatory genes related to Th1 function as compared to the other age groups(Figure 2). At both time points, adult T cells were more likely to produce pro-inflammatory IFN-γ and TNF-α than the infant cells, while Treg cytokines IL-10 and TGF-β1 showed similar production from infants and adults(Figure 3).
Conclusion(s): CD4+ T cells from extremely premature infants were more likely to expand the Treg population following stimulation. Extremely premature T cells had less upregulation and production of pro-inflammatory cytokines, with more production of anti-inflammatory Treg cytokines. This suggests that extremely premature infants tend towards an anti-inflammatory, regulatory adaptive immunity motif, which likely contributes to their increased risk of infection.