Neonatal Neurology: Clinical Research
Neonatal Neurology 5: Clinical
119 - Evolution of the Central Autonomic Network in premature neonates
Sunday, April 30, 2023
3:30 PM - 6:00 PM ET
Poster Number: 119
Publication Number: 119.337
Publication Number: 119.337
Kelsey Christoffel, Children's National Health System, Washington, DC, United States; Josepheen De Asis-Cruz, Childrens National Hospital, Washington, DC, United States; Kushal J. Kapse, Children's National Hospital, Washington, DC, United States; Emma Spoehr, Children's National Health System, Washington, DC, United States; Sudeepta Basu, George Washington University, Washington, DC, United States; Nickie Andescavage, Children's National Health System, Washington, DC, United States; Adre Du Plessis, Children's National Health System, Washington, DC, United States; Catherine Limperopoulos, Children's Hospital, Washington, DC, United States

Kelsey Christoffel, MD (she/her/hers)
Fetal-Neonatal Neurology Fellow
Children's National Health System
Washington, District of Columbia, United States
Presenting Author(s)
Background: The Central Autonomic Network (CAN) is known to influence autonomic nervous system (ANS) function in adults but remains relatively unexplored in the neonatal period. In premature neonates, connectivity studies demonstrate perturbances in connectivity of structures integral to the CAN at term equivalent age (TEA) when compared to term neonates. Furthermore, premature neonates are often born during a window of critical ANS development, providing a unique opportunity to study CAN development in this context.
Objective: To characterize CAN development using resting state functional MRI (rsfMRI) in premature neonates from birth to TEA.
Design/Methods: Ninety-four premature neonates underwent non-sedated rsfMRI at several timepoints between birth and TEA, with a total of 212 total scans. A standardized neonatal brain atlas was used for seed-based approach to define regions of interest (ROIs) pertinent CAN structures, including brainstem, cerebellum, anterior cingulate gyrus, medial prefrontal cortex, precuneus, hippocampus, amygdala, insula, and thalamus. Correlation of blood oxygen level-dependent based signals was assessed between ROIs as a marker of functional connectivity. Postmenstrual age (PMA) was correlated with CAN connectivity for each ROI. Subjects were compared in several groups based on clinical comorbidities using two sample t-tests.
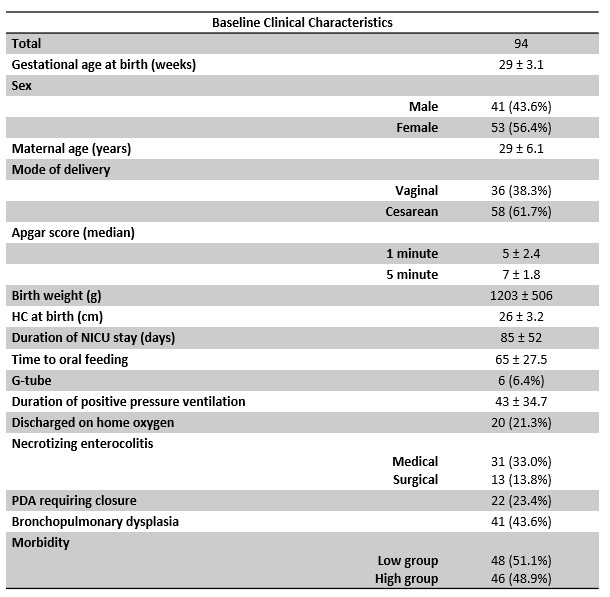
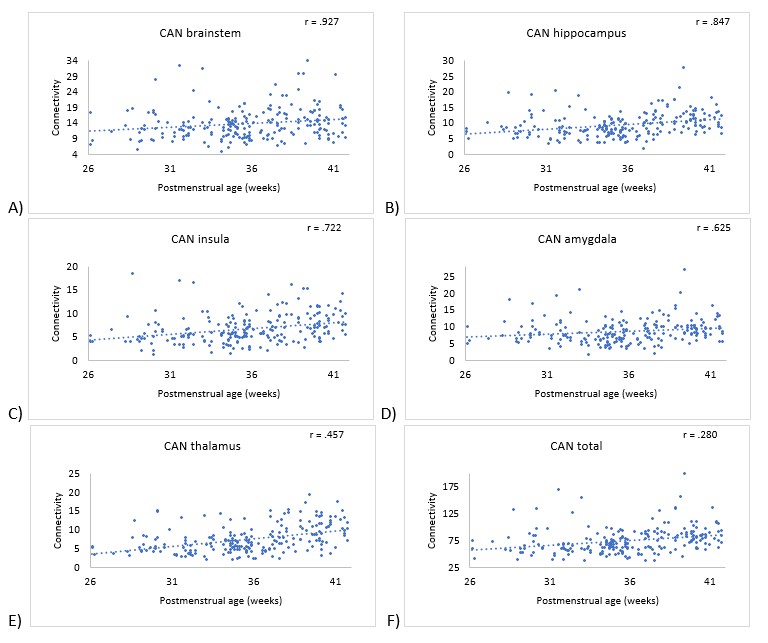
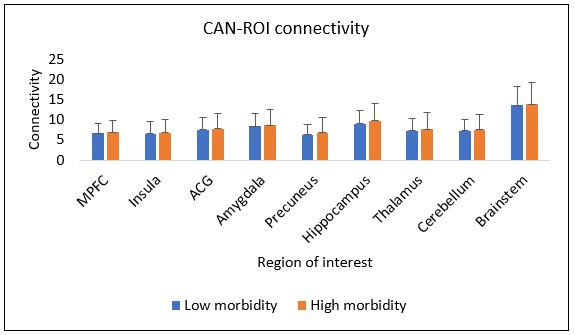
Results: Mean gestational age (GA) at birth for all 94 subjects was 29 (± 3.1 weeks). Significant positive correlations were noted between PMA and regional CAN connectivity for: brainstem (r = .927, p=.01); hippocampus (r = .847, p< .001); insula (r = .722, p < .001); amygdala (r = .625, p=.015); thalamus (r = .457, p< .001); and total CAN connectivity (r = .280, p< .001). CAN connectivity prior to 30 weeks was not significantly associated with subsequent TEA connectivity for any ROI. There was no significant difference in CAN connectivity between high and low morbidity groups at TEA for any ROI, nor were there significant differences based on a diagnosis of necrotizing enterocolitis (NEC) or bronchopulmonary dysplasia (BPD).
Conclusion(s): CAN connectivity increases with PMA at the insula, hippocampus, amygdala, thalamus, and brainstem, suggesting an emerging network and communication between these regions during ex-utero third trimester brain development. Higher connectivity in the early third trimester is not clearly associated with higher term connectivity, suggesting that external environmental factors may influence CAN development. However, our data suggest CAN development is not explained by diagnosis of BPD, NEC, or overall morbidity alone.