Neonatal General
Neonatal General 7: NICU Practices 1
178 - Dexmedetomidine use and association with opioid exposure among preterm infants in United States neonatal intensive care units (NICUs)
Publication Number: 178.331

Ryan Kilpatrick, MD (he/him/his)
Fellow
Duke University School of Medicine
Durham, North Carolina, United States
Presenting Author(s)
Background:
Dexmedetomidine, an alpha-2-agonist, is not approved by the Food and Drug Administration for use in premature infants. However, the off-label use of dexmedetomidine in premature infants has increased 50-fold in the last decade. Currently, there are no large studies characterizing dexmedetomidine use in United States NICUs or examining the impact of dexmedetomidine on opioid use in infants.
Objective:
To describe dexmedetomidine use patterns in the NICU and examine the association between dexmedetomidine and opioid use in premature infants.
Design/Methods:
We performed a multicenter, observational cohort study of inborn infants with gestational age (GA) < 37 weeks discharged between 2010-2020 from Pediatrix Medical Group NICUs. Infants who were transferred, died prior to postnatal day 2, or were born with major congenital anomalies were excluded. We compared demographics and characteristics between infants who did and did not receive dexmedetomidine. We described timing of initiation and duration of dexmedetomidine exposure. We examined trends in dexmedetomidine and opioid use by year and gestational age.
Results:
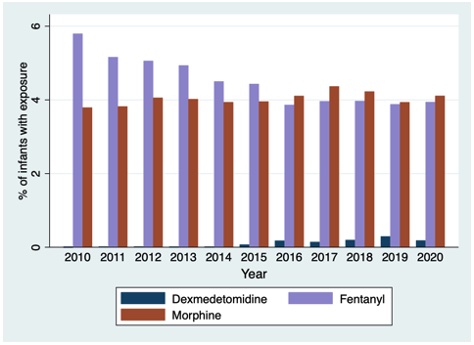
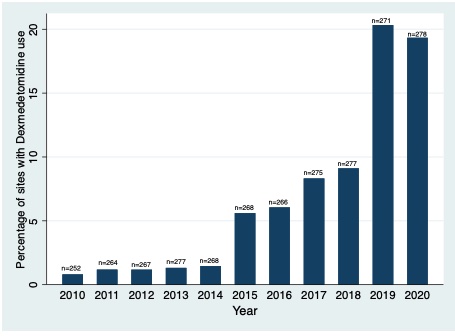
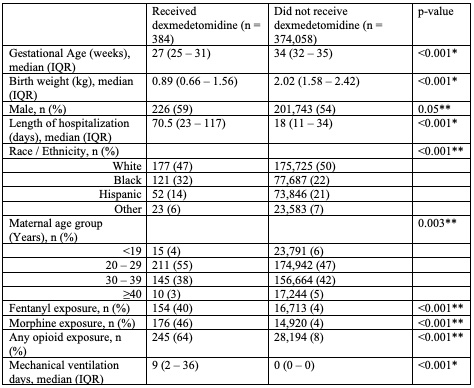
Of 395,122 included infants from 383 centers, 374,442 infants had medication data available. The median (25th-75th percentile) gestational age and birthweight were 34 (32, 35) weeks and 2.04 (1.61, 2.44) kg, respectively. There were 384 (0.1%) infants who received dexmedetomidine. Infants who received dexmedetomidine were born more immature, had lower birthweight, longer length of hospitalization, higher opioid exposure, and more days of mechanical ventilation (Table 1). Dexmedetomidine use increased from 2010 to 2020, while overall opioid exposure decreased, p< 0.001 (Figure 1). Percentage of infants who received fentanyl decreased from 5.8% to 3.9% from 2010 to 2020, while morphine remained relatively stable (3.8% to 4.1%). Median (IQR) duration of dexmedetomidine receipt was 6 days (2, 14). Median (IQR) postnatal age at the time of starting dexmedetomidine was 3 (1, 35) days. Percentage of sites with any dexmedetomidine use increased significantly from 2010 to 2020 (0.8% to 19.3%) (Figure 2).
Conclusion(s):
Dexmedetomidine use in premature infants increased significantly between 2010 – 2020, while overall opioid exposure decreased. Future studies are required to further examine dexmedetomidine’s short- and long-term effects in premature and critically ill infants.