Neonatal Pulmonology
Neonatal Pulmonology 1: Lung Development, Control of Breathing
251 - Novel Peripheral Chemoreceptor Agonist Stimulates Initiation and Maintenance of Breathing in Preterm Lambs
Publication Number: 251.339

George C. Dungan, II, MPhil (Med) (he/him/his)
Adjunct Professor
Canisius College
Denton, Texas, United States
Presenting Author(s)
Background: Apnea and failure to initiate breathing is a common presentation in preterm neonates owing to an immature respiratory control. ENA-001 is a novel agnostic respiratory stimulant, acting on peripheral carotid-body chemoreceptors to drive ventilation, currently being evaluated in adult humans to reverse respiratory depression.
Objective: This study evaluates the efficacy of ENA-001 to stimulate breathing in the immature neural circuitry of a preterm neonatal lamb model.
Design/Methods:
18 neonatal lamb twins (133±2.2d GA) were delivered by c-section from ewes receiving betamethasone 48 and 24 hours pre partum. All lambs received surfactant at delivery, and instrumented prior to initiation of the test article. After arterial and venous cannulation and endotracheal intubation, twin-pairs of lambs were randomized to receive either IV ENA-001 (n=8) or Sham (D5W). ENA-001 was administered as an initial bolus (3mg/kg) followed by an ascending dose continuous infusion over a 4-hours, hourly at 0.4, 1.1, 2.0 and 12.0 mg/kg/hr as 4 mL/kg/hr in D5W. Control animals received 4mL/kg/min D5W. All lambs were sedated as needed for comfort. Vital signs, arterial blood gases, EtCO2, and spirometry were collected every 15 minutes. Lambs without respiratory effort after 30 minutes of resuscitation were deemed unviable and allowed to expire.
Results:
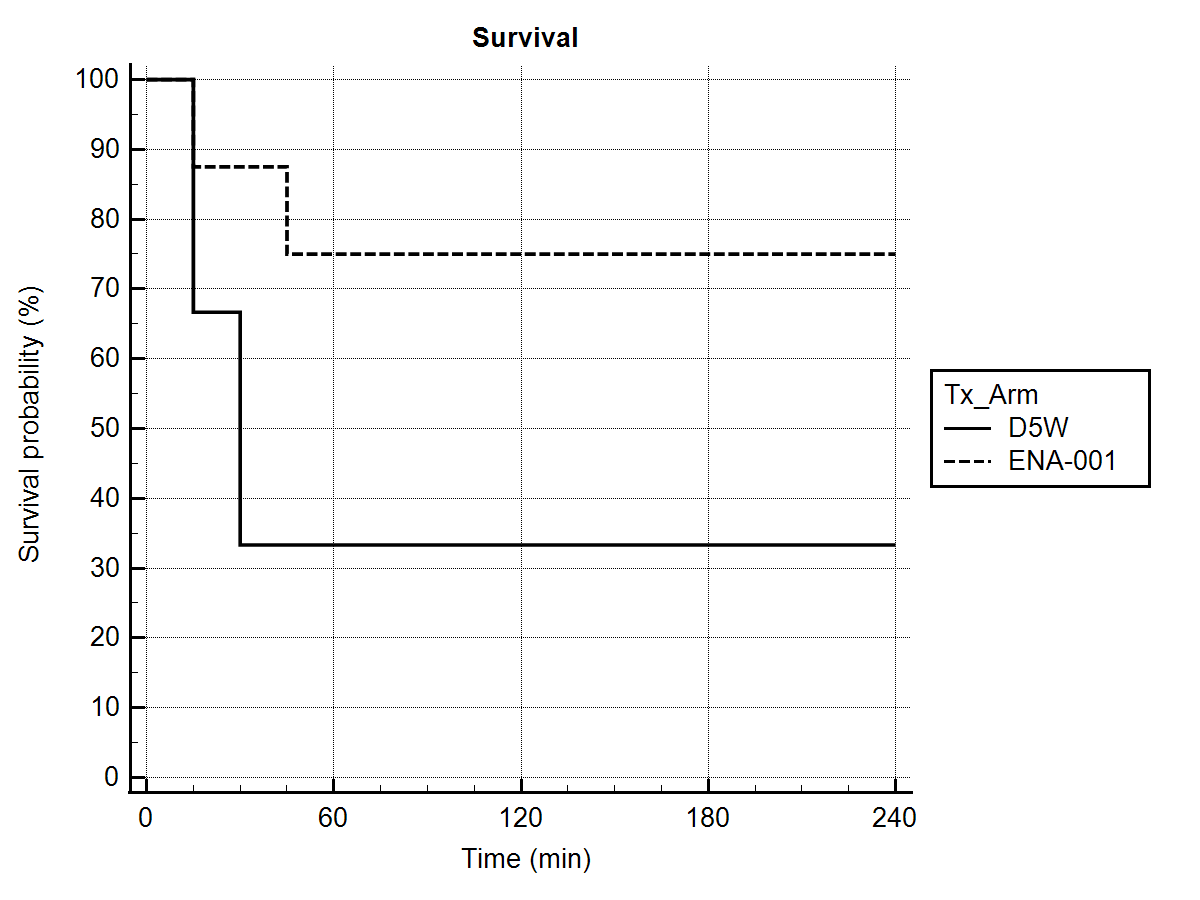
Of 8 lambs receiving ENA-001, 6/8 (75%) survived to 4 hours versus 3/9 (33%) for Sham (Fig. 1). The surviving majority receiving ENA-001 demonstrated breathing performance comparable to that expected for comparable lambs surviving to 4 hours. ENA-001 was associated with a greater tidal volume at higher plasma concentrations, however there was not a similar increase in respiratory rate. The plasma concentration at 1.1 and 2.0 mg/kg/hr ENA-001 dosing was approximately 2000 ng/mL and may suggest an effective dosing target.
Conclusion(s):
ENA-001 was able to stimulate and drive breathing effort to improve mortality outcomes in preterm lambs on the cusp of viability. ENA-001 may play a role in the clinical management of ineffectual respiratory effort in neonates. These data will guide more directed research with ENA-001 to establish effective dose concentrations and interactions with other therapies.