Nephrology: Transplant
Nephrology 4: Transplant
42 - Allosure values in pediatric kidney transplant recipients
Publication Number: 42.349

Arundhati S. Kale, MD (she/her/hers)
Professor of Pediatrics
UC Davis
Sacramento, California, United States
Presenting Author(s)
Background:
AlloSure is a laboratory-developed, clinically-validated, next generation sequencing assay that utilizes single-nucleotide polymorphisms to accurately differentiate and quantify the fraction of donor-derived cell-free DNA (dd-cfDNA) in the plasma of solid organ transplant (tx) recipients without separate genotyping of either the donor or the recipient1. Detection of dd-cfDNA is a marker of tissue injury and may be a sensitive marker of graft injury secondary to rejection.
Objective: Adult data shows a correlation between Allosure (AS) titers and graft injury; few data exist in children. Our objective was to determine the overall AS values in children, determine if any patterns emerged with donor/recipient size mismatch and correlation with biopsy (bx) findings.
Design/Methods: We obtained paired AS samples at least 4 weeks apart on 38 pediatric tx patients (pts). Analysis done to determine:
- AS values in small pts (< 20 kg at time of tx) with adult donors vs size matched donor/recipients
- Correlate AS values with biopsy findings
- Overall AS range in pediatric pts
- whether AS values changed over time in the first year post-tx
Results: Age range of pts was 2-19 years (yr); 16 male and 22 female; post-tx range to first draw was 4 weeks-16 yr. There were 13 white, 5 Black, 10 Hispanic and 10 Asian/mixed ethnicities. All received thymoglobulin induction with tacrolimus and mycophenolate mofetil maintenance immunosuppression. 14 pts had surveillance biopsies with paired Allosure assays. 4 pts were < 20 kg at the time of tx and received adult size kidneys. The mean overall AS value in pts with stable renal function was 0.33. Pts with normal histology on surveillance bx had a mean value of 0.34 vs 0.45 in those that showed borderline changes. Mean AS value was 0.46 in pts with size matched kidneys vs 0.67 in those < 20 kg. AS decreased from 0.49 to 0.18 when measured serially in the 1st year post-tx.
Conclusion(s):
Data from pediatric pts matches that reported in prior studies (mean value 0.33)
There was no statistical difference between AS values and biopsies showing "no rejection" vs "borderline changes"- AS decreased from 0.49 to 0.18 in serial measurements in the 1st year post-tx
- Data from 4 pts who were < 20 kg at the time of tx with adult kidneys showed no statistical difference compared to pts with size matched kidneys
- Limitations of the study are mainly small numbers in each group, given it is from a single center.
1. Grskovic, M., et al., Validation of a Clinical-Grade Assay to Measure Donor-Derived Cell-Free DNA in Solid Organ Transplant Recipients. J Mol Diagn, 2016. 18(6)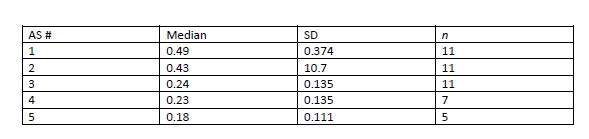
.jpg)
.jpg)
