Neonatal/Infant Resuscitation
Neonatal/Infant Resuscitation 2
323 - Colorimetric CO2 Detector To Improve Effective Ventilations: A pilot Randomized Controlled Study
Publication Number: 323.348
- JK
Juin Yee Kong, MD (she/her/hers)
Senior Consultant
Kk women’s and children’s hospital
Singapore, Singapore
Presenting Author(s)
Background: Up to 10% of delivery room (DR) resuscitations in newborn involve mask positive pressure ventilation (mPPV). Establishing a patent airway, especially the preterms may be challenging given their small size and unique airway anatomy. Current Neonatal Resuscitation Program (NRP) recommends for assessment of effective assisted ventilation by increase in heart rate(HR), oxygen saturation(SpO2) levels, and adequacy of chest rise. This may be difficult in a crowded setting with inter-observer variability. Video resuscitation studies described the use of colorimetric ETCO2 detectors during mPPV to facilitate recognition and management of obstructed airway by guidance of colour change.
Objective: To investigate the value of a cost effective, colorimetric ETCO2 detector in guiding the resuscitation team towards providing effective mPPV on preterm infants in the DR.
Design/Methods:
This is a pilot randomized controlled trial. Preterm infants < 32 weeks gestation who require mPPV after birth were randomized into control group versus monitor group. In the monitor group, ETCO2 detector is attached to facemask to guide mPPV by observation of colour change when breaths were effective. In the control group, routine assessment of effective breaths as per NRP guidelines was practiced. Physiologic data, respiratory data and video recordings were collected. Primary outcome is defined as combined duration of bradycardia (HR< 100bpm) and desaturation (SpO2 levels below recommended target). Secondary outcomes were need for DR interventions. Statistical analysis was performed using SPSS.
Results:
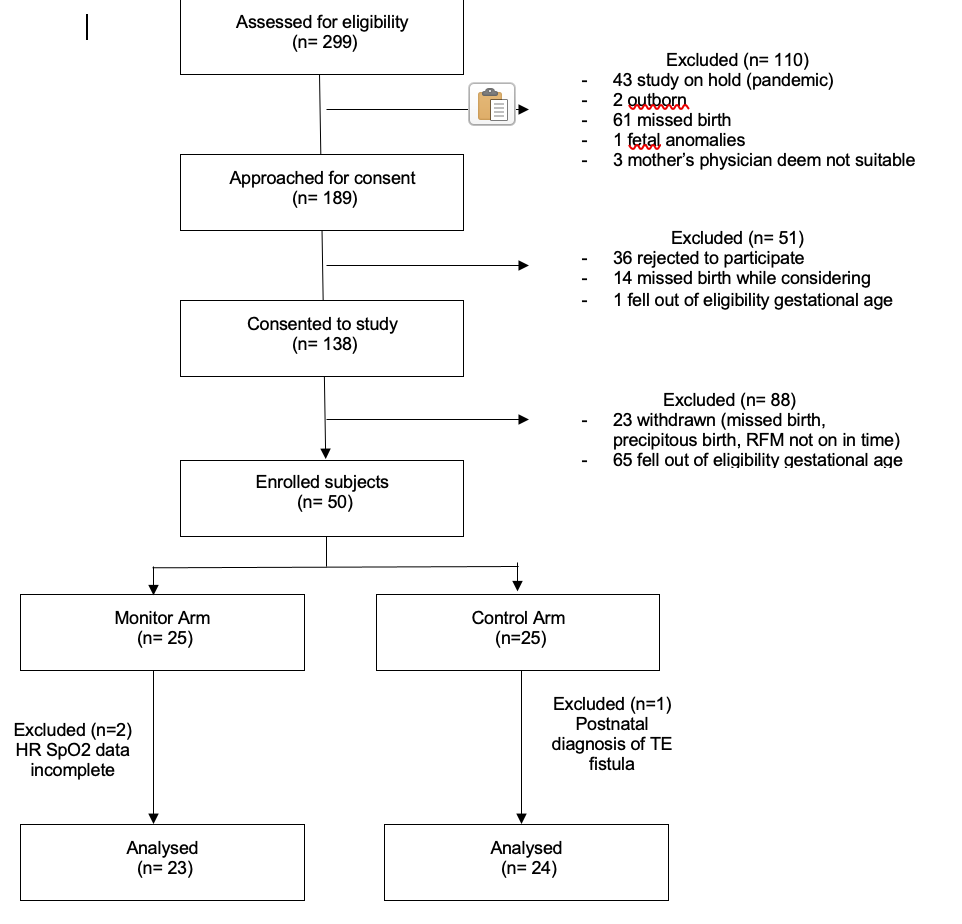
A convenient sample of 50 patients were enrolled in the study from 6 Jun 2019 to 15 Feb 2021. Data for 47 subjects were available for analysis. Birthweight and gestational age were similar in both groups. Median BDI for both groups were 196s [28,235] in monitor group vs 188s [74,349]in control group, p=0.44. Incidence of intubation, chest compression and epinephrine administration in the DR were not statistically different. There were no DR deaths in the study cohort.
Conclusion(s): When using a qualitative ETCO2 detector as a guide for airway potency during mPPV in preterm newborns, no statistically difference was observed in the duration of bradycardia and desaturations below oxygen target range. Future large statistical powered studies are needed to investigate the effect of qualitative EtCO2 especially in populations who might benefit the most, such as those depressed at birth who require more assisted ventilation.
.png)
.png)
